LENLI CAPSULES 5MG
4.1 Therapeutic indications
Multiple myeloma
Lenalidomide as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.
Lenalidomide as combination therapy with dexamethasone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.
Lenalidomide in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.
4.3 Contraindications
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
- Women who are pregnant.
- Women of childbearing potential unless all of the conditions of the Pregnancy Prevention Programme are met (see sections 4.4 and 4.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.2 Posology and method of administration
Lenalidomide treatment should only be prescribed by Specialist Physician experienced in the management of malignancies, who have undergone the Lenli educational programme on Pregnancy Prevention Programme.
Treatment must be initiated and monitored under the supervision of physicians experienced in the management of multiple myeloma (MM).
For the indication described below:
- Dose is modified based upon clinical and laboratory findings (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
- Dose adjustments, during treatment and restart of treatment, are recommended to manage grade 3 or 4 thrombocytopenia, neutropenia, or other grade 3 or 4 toxicity judged to be related to lenalidomide.
- In case of neutropenia, the use of growth factors in patient management should be considered.
- If less than 12 hours has elapsed since missing a dose, the patient can take the dose. If more than 12 hours has elapsed since missing a dose at the normal time, the patient should not take the dose, but take the next dose at the normal time on the following day.
Posology
Newly diagnosed multiple myeloma (NDMM)
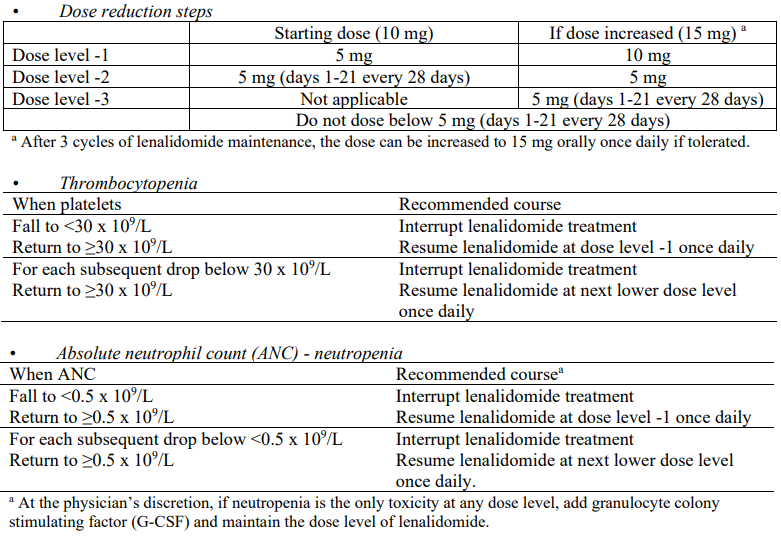
Lenalidomide maintenance in patients who have undergone autologous stem cell transplantation (ASCT)
Lenalidomide maintenance should be initiated after adequate haematologic recovery following ASCT in patients without evidence of progression. Lenalidomide must not be started if the Absolute Neutrophil Count (ANC) is <1.0 x 109/L, and/or platelet counts are <75 x 109/L.
Recommended dose
The recommended starting dose is lenalidomide 10 mg orally once daily continuously (on days 1 to 28 of repeated 28-day cycles) given until disease progression or intolerance. After 3 cycles of lenalidomide maintenance, the dose can be increased to 15 mg orally once daily if tolerated.
Lenalidomide in combination with dexamethasone until disease progression in patients who are not eligible for transplant
Lenalidomide treatment must not be started if the ANC is <1.0 x 109/L, and/or platelet counts are <50 x 109/L.
Recommended dose
The recommended starting dose of lenalidomide is 25 mg orally once daily on days 1 to 21 of repeated 28-day cycles.
The recommended dose of dexamethasone is 40 mg orally once daily on days 1, 8, 15 and 22 of repeated 28-day cycles. Patients may continue lenalidomide and dexamethasone therapy until disease progression or intolerance.
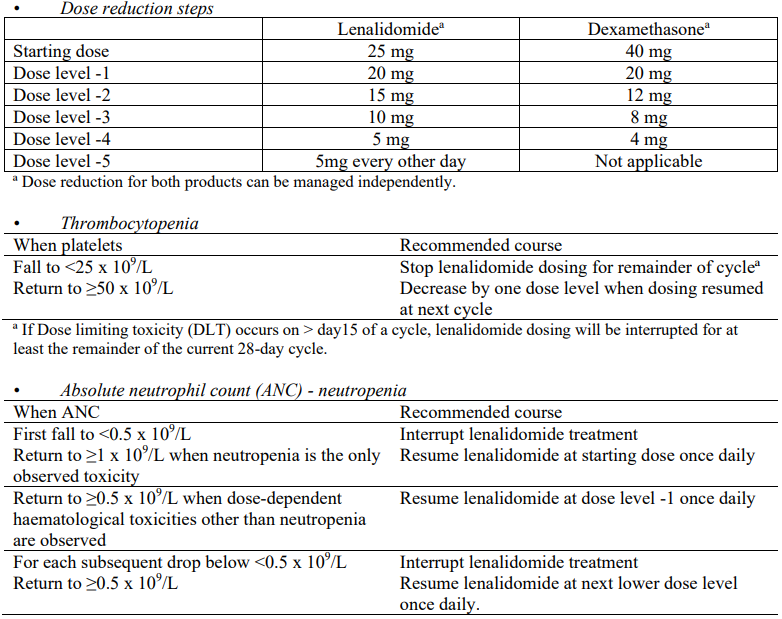
For hematologic toxicity the dose of lenalidomide may be re-introduced to the next higher dose level (up to the starting dose) upon improvement in bone marrow function (no hematologic toxicity for at least 2 consecutive cycles: ANC ≥1.5 x 109/L with a platelet count ≥100 x 109/L at the beginning of a new cycle).
Multiple myeloma with at least one prior therapy
Lenalidomide treatment must not be started if the ANC <1.0 x 109/L, and/or platelet counts <75 x 109/L or, dependent on bone marrow infiltration by plasma cells, platelet counts <30 x 109/L.
Recommended dose
The recommended starting dose of lenalidomide is 25 mg orally once daily on days 1 to 21 of repeated 28-day cycles. The recommended dose of dexamethasone is 40 mg orally once daily on days 1 to 4, 9 to 12, and 17 to 20 of each 28-day cycle for the first 4 cycles of therapy and then 40 mg once daily on days 1 to 4 every 28 days.
Prescribing physicians should carefully evaluate which dose of dexamethasone to use, taking into account the condition and disease status of the patient.
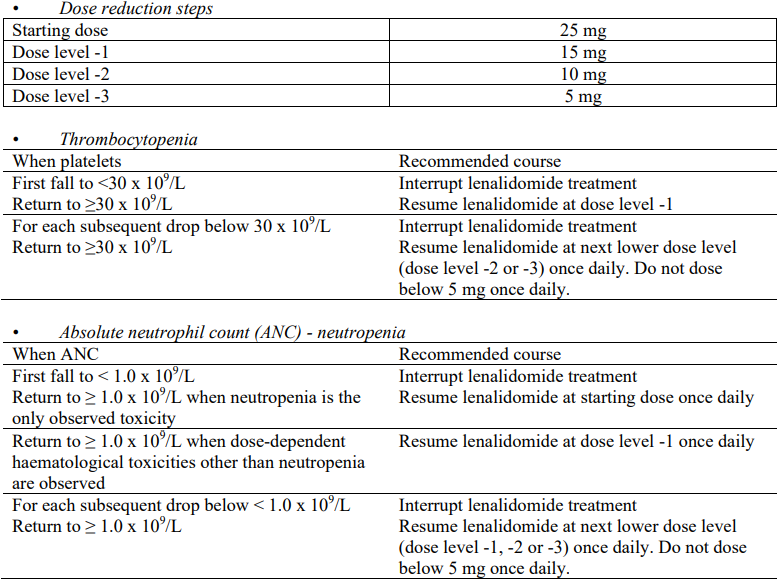
All indications
For other grade 3 or 4 toxicities judged to be related to lenalidomide, treatment should be stopped and only restarted at next lower dose level when toxicity has resolved to ≤ grade 2 depending on the physician’s discretion.
Lenalidomide interruption or discontinuation should be considered for grade 2 or 3 skin rash.
Lenalidomide must be discontinued for angioedema, grade 4 rash, exfoliative or bullous rash, or if Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) or Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) is suspected, and should not be resumed following discontinuation from these reactions.
Special populations
Paediatric population
Lenalidomide should not be used in children and adolescents from birth to less than 18 years because of safety concerns (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Elderly
Currently available pharmacokinetic data are described in section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information. Lenalidomide has been used in clinical trials in multiple myeloma patients up to 91 years of age (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it would be prudent to monitor renal function.
Newly diagnosed multiple myeloma: patients who are not eligible for transplant
Patients with newly diagnosed multiple myeloma aged 75 years and older should be carefully assessed before treatment is considered (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
For patients older than 75 years of age treated with lenalidomide in combination with dexamethasone, the starting dose of dexamethasone is 20 mg once daily on days 1, 8, 15 and 22 of each 28-day treatment cycle.
In patients with newly diagnosed multiple myeloma aged 75 years and older who received lenalidomide, there was a higher incidence of serious adverse reactions and adverse reactions that led to treatment discontinuation.
Lenalidomide combined therapy was less tolerated in newly diagnosed multiple myeloma patients older than 75 years of age compared to the younger population. These patients discontinued at a higher rate due to intolerance (Grade 3 or 4 adverse events and serious adverse events), when compared to patients <75 years.
Multiple myeloma: patients with at least one prior therapy
The percentage of multiple myeloma patients aged 65 or over was not significantly different between the lenalidomide/dexamethasone and placebo/dexamethasone groups. No overall difference in safety or efficacy was observed between these patients and younger patients, but greater pre-disposition of older individuals cannot be ruled out.
Patients with renal impairment
Lenalidomide is primarily excreted by the kidney; patients with greater degrees of renal impairment can have impaired treatment tolerance (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). Care should be taken in dose selection and monitoring of renal function is advised.
No dose adjustments are required for patients with mild renal impairment.
The following dose adjustments are recommended at the start of therapy and throughout treatment for patients with moderate or severe impaired renal function or end stage renal disease.
There are no phase III trial experiences with End Stage Renal Disease (ESRD) (CLcr <30 mL/min, requiring dialysis).
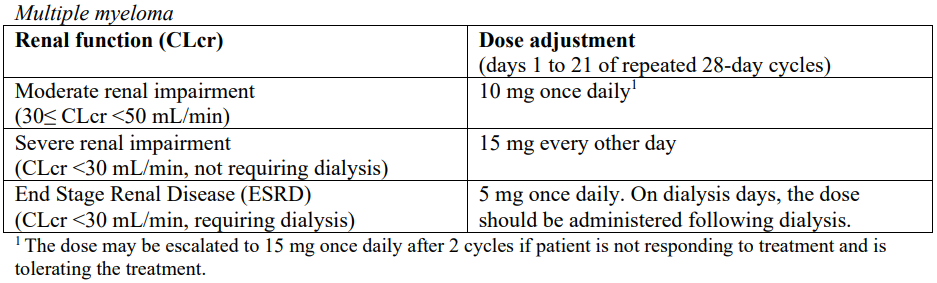
After initiation of lenalidomide therapy, subsequent lenalidomide dose modification in renally impaired patients should be based on individual patient treatment tolerance, as described above.
Patients with hepatic impairment
Lenalidomide has not formally been studied in patients with impaired hepatic function and there are no specific dose recommendations.
Method of administration
Oral use.
Lenalidomide capsules should be taken orally at about the same time on the scheduled days. The capsules should not be opened, broken or chewed. The capsules should be swallowed whole, preferably with water, either with or without food.
It is recommended to press only on one end of the capsule to remove it from the blister thereby reducing the risk of capsule deformation or breakage.