REMERON SOLTAB 15 mg
4.1 Therapeutic indications
Episode of major depression.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients.
Concomitant use of mirtazapine with monoamine oxidase (MAO) inhibitors (see section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.2 Posology and method of administration
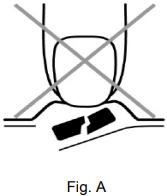
In order to prevent crushing the tablet, do not push against the tablet pocket (Figure A).
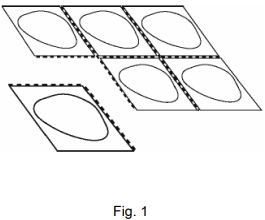
Each strip contains six tablet pockets, which are separated by perforations. Tear off one tablet pocket along the dotted lines (Figure 1).
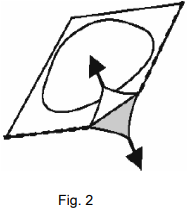
Carefully peel off the lidding foil, starting in the corner indicated by the arrow (Figures 2 and 3).
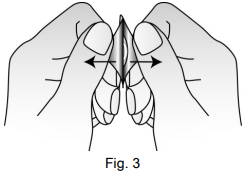
The tablet should be taken out of the strip with dry hands and should be placed on the tongue (Figure 4). The tablet will rapidly disintegrate and can be swallowed without water.
Adults
Treatment should begin with 15 mg daily. The dosage generally needs to be increased to obtain an optimal clinical response. The effective daily dose is usually between 15 and 45 mg (the dose should be taken at night).
Mirtazapine begins to exert its effect in general after 1–2 weeks of treatment. Treatment with an adequate dose should result in a positive response within 2–4 weeks. With an insufficient response, the dose can be increased up to the maximum dose. If there is no response within a further 2–4 weeks, then treatment should be stopped.
Elderly
The recommended dose is the same as that for adults. In elderly patients an increase in dosing should be done under close supervision to elicit a satisfactory and safe response.
Children and adolescents under the age of 18 years
Remeron should not be used in children and adolescents under the age of 18 years (see section 4.4) as efficacy was not demonstrated in two short-term clinical trials (see section 5.1) and because of safety concerns (see sections 4.4, 4.8 and 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Renal impairment
The clearance of mirtazapine may be decreased in patients with moderate to severe renal impairment (creatinine clearance < 40 ml/min). This should be taken into account when prescribing Remeron to this category of patients (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic impairment
The clearance of mirtazapine may be decreased in patients with hepatic impairment. This should be taken into account when prescribing Remeron to this category of patients, particularly with severe hepatic impairment, as patients with severe hepatic impairment have not been investigated (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Mirtazapine has an elimination half-life of 20–40 hours and therefore Remeron is suitable for once daily administration. It should be taken preferably as a single night-time dose before going to bed. Remeron may also be given in two divided doses (once in the morning and once at night-time; the higher dose should be taken at night).
The tablets should be taken orally. The tablet will rapidly disintegrate and can be swallowed without water.
Patients with depression should be treated for a sufficient period of at least 6 months to ensure that they are free from symptoms.
It is recommended to discontinue treatment with mirtazapine gradually to avoid withdrawal symptoms (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).