GENOTROPIN FOR INJECTION 16 iu (5.3 mg) /ml
4.1 Therapeutic indications
Children
Growth disturbance due to insufficient secretion of growth hormone and growth disturbance associated with Turner syndrome.
Adults
Replacement therapy in adults with pronounced growth hormone deficiency.
4.3 Contraindications
Somatropin is contraindicated in patients who have evidence of neoplastic activity and in patients with uncontrolled growth of benign intracranial tumors. Anti-tumor therapy must be completed prior to starting somatropin.
Somatropin is contraindicated in patients with acute critical illness due to complications following open heart or abdominal surgery, multiple accidental trauma, or acute respiratory failure. Two placebo-controlled clinical trials (N=522), conducted in adult patients to evaluate the effects of somatropin 5.3 or 8 mg (16 or 24 international units) on length of stay in intensive care units, showed significantly higher mortality (41.9% vs. 19.3%) in patients treated with somatropin compared with those who received placebo (see section 4.4 Special warnings and precautions for use in patients who are receiving somatropin for growth hormone replacement – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hypersensitivity to the active substance or to any of the excipients.
4.2 Posology and method of administration
The dosage and administration schedule should be individualized. Somatropin should be given subcutaneously and the injection site varied to prevent lipoatrophy.
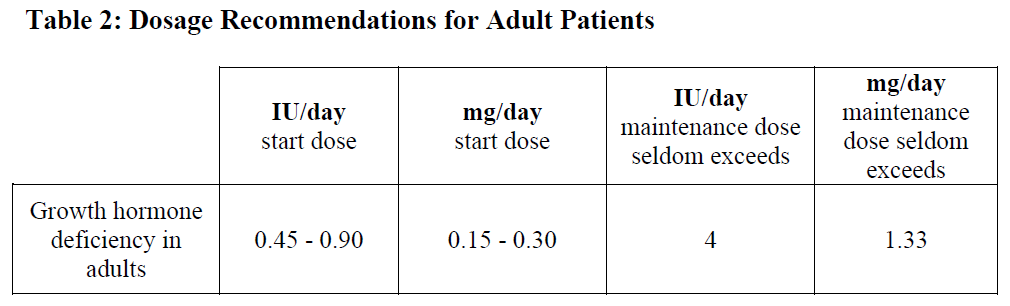
Growth disturbance due to insufficient secretion of growth hormone in children: Generally, a dose of 0.07–0.10 international units/kg (0.025–0.035 mg/kg) body weight per day or 2.1–3.0 international units/m2 (0.7–1.0 mg/m2) body surface area per day is recommended. Even higher doses have been used.
Growth disturbance due to Turner syndrome: A dose of 0.14 international units/kg (0.045–0.050 mg/kg) body weight per day or 4.3 international units/m2 (1.4 mg/m2) body surface area per day is recommended.
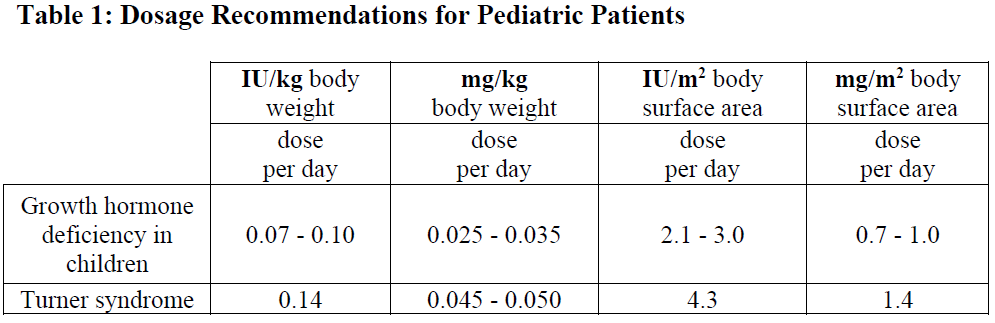
Growth hormone deficient adult patients: The recommended starting dose is 0.45 – 0.90 international units (0.15 – 0.30 mg) per day. The final dose should be individually titrated as needed with respect to age and gender. The daily maintenance dose seldom exceeds 4 international units (1.33 mg) per day. Women may require higher doses than men. This means that there is a risk that women, especially those on oral oestrogen replacement may be under-treated. As normal physiological growth hormone production decreases with age, dose requirements may be reduced. Clinical response, side effects, and determination of IGF-I in serum may be used as guidance for dose titration.