GIOTRIF FILM-COATED TABLETS 40MG
4.1 Therapeutic Indications
- GIOTRIF is indicated for the first line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with Epidermal Growth Factor Receptor (EGFR) mutation(s).
- GIOTRIF as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC of squamous histology progressing on or after platinum-based chemotherapy.
4.3 Contraindications
GIOTRIF is contraindicated in patients with known hypersensitivity to afatinib or to any of the excipients.
4.2 Dosage and Administration
The recommended dose of GIOTRIF is 40 mg orally once daily.
GIOTRIF should be taken without food. Food should not be consumed for at least 3 hours before and at least 1 hour after taking GIOTRIF (see sections Interactions and Pharmacokinetics – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). Tablets should be swallowed whole with water.
GIOTRIF treatment should be continued until disease progression or until no longer tolerated by the patient (see Table 1 below).
Dose adjustment for adverse reactions
Symptomatic adverse drug reactions (e.g. severe/persistent diarrhoea or skin related adverse reactions) may be successfully managed by treatment interruption and dose reductions of GIOTRIF as outlined in Table 1 (see section Adverse Reactions; for further details on management of specific drug related Adverse Events (AEs) see section Special Warnings and Precautions – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
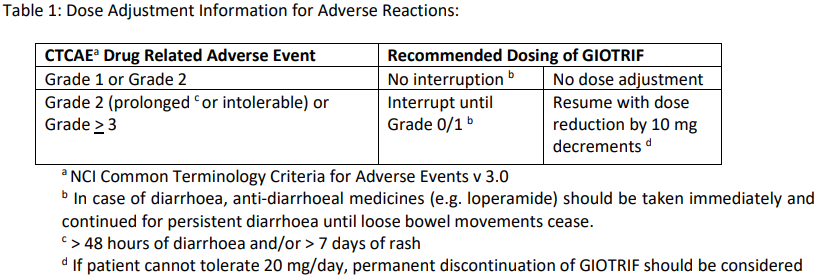
Interstitial Lung Disease (ILD) should be considered if a patient develops acute or worsening of respiratory symptoms in which case GIOTRIF should be interrupted pending evaluation. If ILD is diagnosed, GIOTRIF should be discontinued and appropriate treatment instituted as necessary [see section Special Warnings and Precautions – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information].
Missed dose
If a dose of GIOTRIF is missed, it should be taken during the same day as soon as the patient remembers. However, if the next scheduled dose is due within 8 hours then the missed dose must be skipped.
Special populations
Patients with renal impairment
Exposure to afatinib was found to be increased in patients with moderate or severe renal impairment (see section Pharmacokinetics – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). Adjustments to the starting dose are not necessary in patients with mild, moderate or severe (eGFR 15–29 mL/min/1.73 m2) renal impairment. Monitor patients with severe renal impairment and adjust GIOTRIF dose if not tolerated. GIOTRIF treatment in patients with eGFR <15 mL/min/1.73 m2 or on dialysis is not recommended.
Patients with hepatic impairment
Exposure to afatinib is not significantly changed in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment (see section Pharmacokinetics – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). Adjustments to the starting dose are not necessary in patients with mild or moderate hepatic impairment. GIOTRIF has not been studied in patients with severe (Child Pugh C) hepatic impairment. GIOTRIF treatment in this population is not recommended.
Age, Race, Gender
No dose adjustment is necessary based on patient age, race, or gender (see section Pharmacokinetics – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric population
The safety and efficacy of GIOTRIF have not been established in paediatric patients.
Treatment of children or adolescents with GIOTRIF was not supported by a clinical trial conducted in paediatric patients and is therefore not recommended.
Use of P-glycoprotein (P-gp) inhibitors
If P-gp inhibitors need to be taken, they should be administered using staggered dosing, i.e. the P-gp inhibitor dose should be taken as far apart in time as possible from the GIOTRIF dose. This means preferably 6 hours (for P-gp inhibitors dosed twice daily) or 12 hours (for P-gp inhibitors dosed once daily) apart from GIOTRIF (see sections Special Warnings and Precautions, Interactions, and Pharmacokinetics – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Alternative method of administration
If dosing of whole tablets is not possible, GIOTRIF tablets can be dispersed in approximately 100 ml of noncarbonated drinking water. No other liquids should be used. The tablet should be dropped into the water without crushing it, and stirred occasionally for up to 15 min until the tablet is broken up into very small particles. The dispersion should be consumed immediately. The glass should be rinsed with approximately 100 ml of water which should also be consumed. The dispersion can also be administered through a gastric tube.