Emend® I.V. Powder for Solution for Infusion 150mg
IV. INDICATIONS
EMEND I.V. is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of:
- highly emetogenic cancer chemotherapy (see DOSAGE AND ADMINISTRATION)
- moderately emetogenic cancer chemotherapy (see DOSAGE AND ADMINISTRATION).
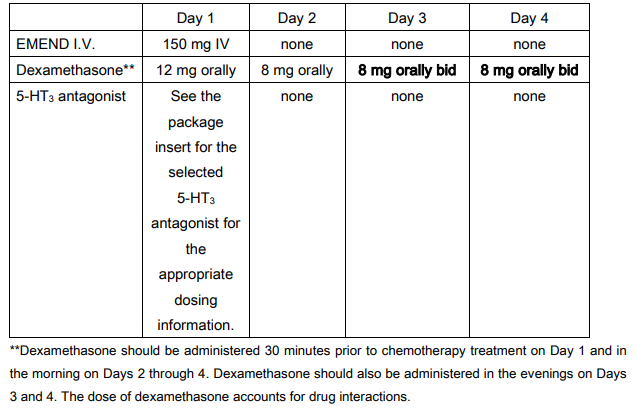
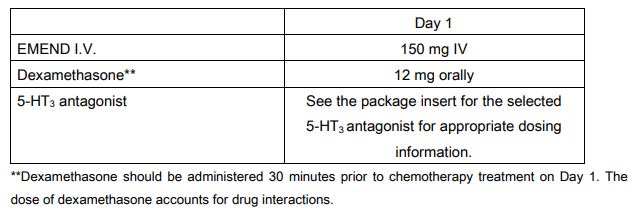
EMEND I.V. should be given in combination with a corticosteroid and a 5-HT3 antagonist.
VII. CONTRAINDICATIONS
EMEND I.V. is contraindicated in patients who are hypersensitive to EMEND I.V., aprepitant, polysorbate 80 or any other components of the product.
EMEND I.V. should not be used concurrently with pimozide, terfenadine, astemizole, or cisapride. Inhibition of cytochrome P450 isoenzyme 3A4 (CYP3A4) by aprepitant could result in elevated plasma concentrations of these drugs, potentially causing serious or life-threatening reactions (see DRUG INTERACTIONS – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
V. DOSAGE AND ADMINISTRATION
EMEND I.V. for intravenous administration is a lyophilized prodrug of aprepitant (EMEND) containing polysorbate 80 (PS80).
EMEND I.V. 150 mg
EMEND I.V. 150 mg is administered on Day 1 as an infusion over 20 – 30 minutes initiated approximately 30 minutes prior to chemotherapy. EMEND I.V. should be administered in conjunction with a corticosteroid and a 5-HT3 antagonist as specified in the tables below. The package insert for the co-administered 5-HT3 antagonist must be consulted prior to initiation of treatment with EMEND I.V. 150 mg.
Recommended dosing for the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy:
Recommended dosing for the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy:
Preparation of EMEND I.V. for Injection 150 mg
- Aseptically inject 5 ml 0.9% saline into the vial. Assure that saline is added to the vial along the vial wall in order to prevent foaming. Swirl the vial gently. Avoid shaking and jetting saline into the vial.
- Aseptically prepare an infusion bag filled with 145 ml of saline.
- Aseptically withdraw the entire volume from the vial and transfer it into an infusion bag containing 145 ml of saline to yield a total volume of 150 ml. Gently invert the bag 2–3 times.
The reconstituted solution should be used immediately; although the reconstituted final drug solution is stable for 24 hours at ambient room temperature (at or below 25°C).
Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit.
EMEND I.V. is incompatible with any solutions containing divalent cations (e.g., Ca2+, Mg2+), including Hartman’s and Lactated Ringer’s Solution. EMEND I.V. must not be reconstituted or mixed with solutions for which physical and chemical compatibility have not been established.
GENERAL INFORMATION
See DRUG INTERACTIONS for additional information on the administration of EMEND I.V. with corticosteroids – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
Refer to the full prescribing information for coadministered antiemetic agents.
No dosage adjustment is necessary based on age, gender, race or Body Mass Index (BMI).
No dosage adjustment is necessary for patients with severe renal insufficiency (creatinine clearance <30 ml/min) or for patients with end stage renal disease undergoing hemodialysis.
No dosage adjustment is necessary for patients with mild to moderate hepatic insufficiency (Child-Pugh score 5 to 9). There are no clinical data in patients with severe hepatic insufficiency (Child-Pugh score >9).