ALUNBRIG FILM-COATED TABLET 90mg
4.1 Therapeutic Indications
ALUNBRIG® is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) previously not treated with an ALK inhibitor.
ALUNBRIG® is indicated for the treatment of adult patients with ALK-positive advanced NSCLC previously treated with crizotinib.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
4.2 Posology and Method of Administration
ALK Testing
A validated ALK assay is necessary for the selection of ALK-positive NSCLC patients. ALK-positive NSCLC status should be established prior to initiation of ALUNBRIG® therapy.
Dosage
The recommended starting dose of ALUNBRIG® is 90 mg once daily for the first 7 days, then 180 mg once daily.
Treatment should continue as long as clinical benefit is observed.
If a dose of ALUNBRIG® is missed or vomiting occurs after taking a dose, an additional dose should not be administered and the next dose of ALUNBRIG® should be taken at the scheduled time.
Dose Adjustments
Dosing interruption and/or dose reduction may be required based on individual safety and tolerability.
ALUNBRIG® dose reduction levels are summarised in Table 1.
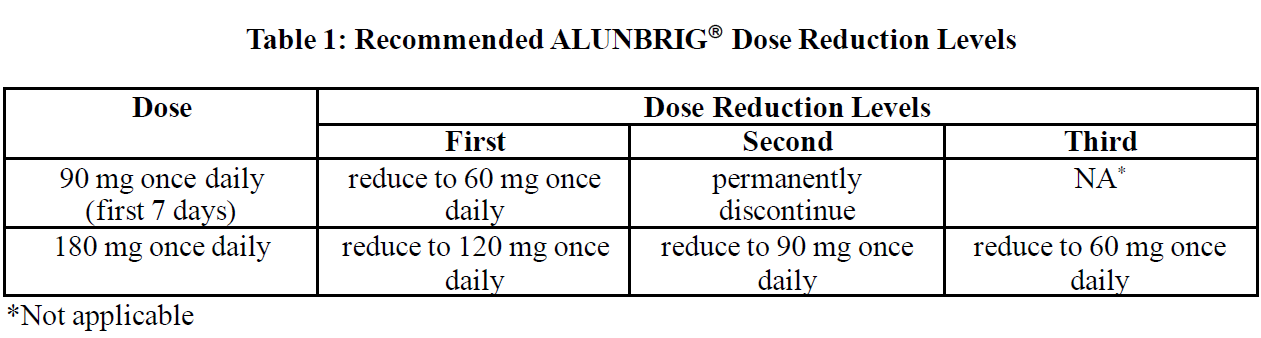
Permanently discontinue ALUNBRIG® if patient is unable to tolerate the 60 mg once daily dose.
If ALUNBRIG® is interrupted for 14 days or longer for reasons other than adverse reactions, treatment should be resumed at 90 mg once daily for 7 days before increasing to the previously tolerated dose.
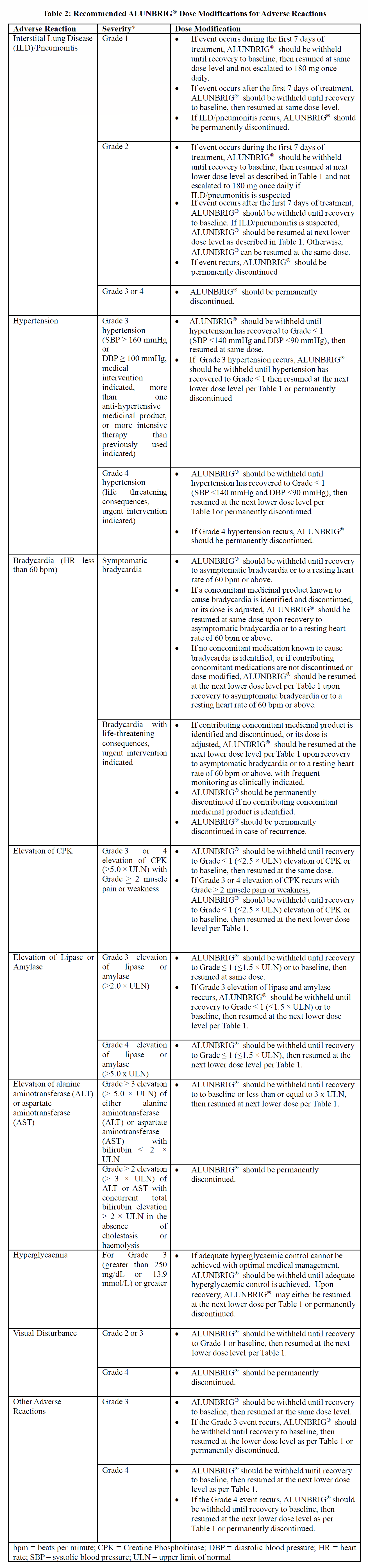
Recommendations for dose modifications of ALUNBRIG® for the management of adverse reactions are summarized in Table 2.
Special Patient Populations
Elderly Patients
The limited data on the safety and efficacy of ALUNBRIG® in patients aged 65 years and older suggest that a dose adjustment is not required in elderly patients (see ACTION AND CLINICAL PHARMACOLOGY – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). There are limited data on patients over 85 years of age.
Pediatric Patients
The safety and efficacy of ALUNBRIG® in patients less than 18 years of age have not been established. No data are available.
Impaired Renal Function
No dose adjustment is recommended for patients with mild or moderate renal impairment (creatinine clearance ≥ 30 mL/min). The dose of Alunbrig should be reduced by approximately 50% (e.g., from 180 mg to 90 mg, or from 90 mg to 60 mg) for patients with severe renal impairment (eGFR < 30 mL/min) (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). Patients with severe renal impairment should be closely monitored for new or worsening respiratory symptoms that may indicate ILD/pneumonitis (e.g., dyspnoea, cough, etc.) particularly in the first week. (see ACTION AND CLINICAL PHARMACOLOGY – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Impaired Hepatic Function
No dose adjustment of Alunbrig is required for patients with mild hepatic impairment (Child-Pugh class A) or moderate hepatic impairment (Child-Pugh class B). The dose of Alunbrig should be reduced by approximately 40% (e.g., from 180 mg to 120 mg, 120 mg to 90 mg, or from 90 mg to 60 mg) for patients with severe hepatic impairment (Child-Pugh class C) . (see ACTION AND CLINICAL PHARMACOLOGY – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Method of Administration
ALUNBRIG® is for oral use. The tablets should be swallowed whole and with water. Do not crush or chew tablets.
ALUNBRIG® may be taken with or without food.