AKYNZEO CAPSULES 300MG/0.5MG
1 INDICATIONS AND USAGE
AKYNZEO is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
4 CONTRAINDICATIONS
None.
2 DOSAGE AND ADMINISTRATION
Administration
One AKYNZEO capsule administered approximately 1 hour prior to the start of chemotherapy.
Highly Emetogenic Chemotherapy, including Cisplatin Based Chemotherapy
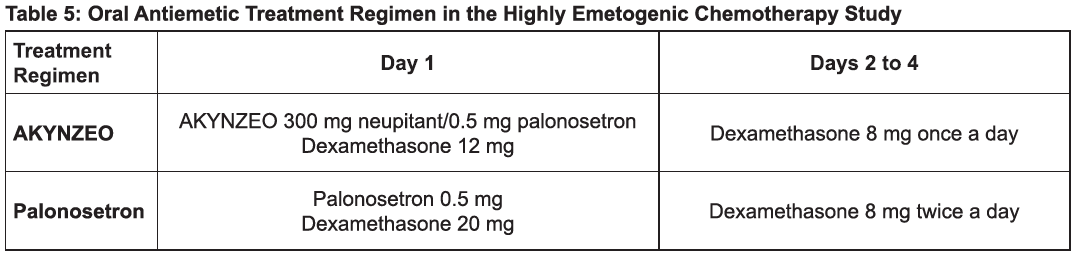
The recommended dosage in adults is one capsule of AKYNZEO administered approximately 1 hour prior to the start of chemotherapy with dexamethasone 12 mg administered orally 30 minutes prior to chemotherapy on day 1 and 8 mg orally once daily on days 2 to 4 [see Clinical Studies (14), Table 5) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ].
[From 14 CLINICAL STUDIES]
Anthracyclines and Cyclophosphamide Based Chemotherapy and Chemotherapy Not Considered Highly Emetogenic
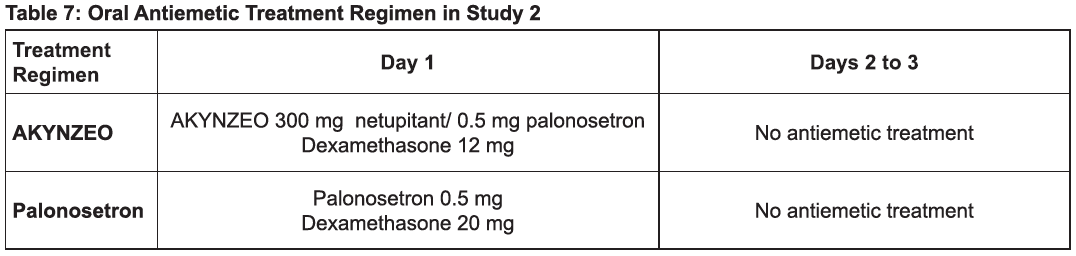
The recommended dosage in adults is one capsule of AKYNZEO approximately 1 hour prior to the start of chemotherapy with dexamethasone 12 mg administered orally 30 minutes prior to chemotherapy on day 1. Administration of dexamethasone on days 2 to 4 is not necessary [see Clinical Studies (14), Table 7 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ].
[From 14 CLINICAL STUDIES]
AKYNZEO can be taken with or without food.