MAVIRET FILM-COATED TABLET 100MG/40MG
4.1 Therapeutic indications
Maviret is indicated for the treatment of chronic hepatitis C virus (HCV) infection in adults and adolescents 12 years and older (see sections 4.2, 4.4. and 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.3 Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
Patients with moderate or severe hepatic impairment (Child-Pugh B or C) or those with any history of prior hepatic decompensation (see sections 4.2, 4.4, and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Concomitant use with atazanavir containing products, atorvastatin, simvastatin, dabigatran etexilate, ethinyl oestradiol-containing products, strong P-gp and CYP3A inducers (e.g., rifampicin, carbamazepine, St. John’s wort ( Hypericum perforatum), phenobarbital, phenytoin, and primidone) (see section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.2 Posology and method of administration
Maviret treatment should be initiated and monitored by a physician experienced in the management of patients with HCV infection.
Posology
Recommended dosage in adults and adolescents 12 years and older
The recommended dose of Maviret is 300 mg/120 mg (three 100 mg glecaprevir /40 mg pibrentasvir tablets), taken orally, once daily at the same time with food (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
The recommended Maviret treatment durations for HCV genotype 1, 2, 3, 4, 5, or 6 infected patients with compensated liver disease (with or without cirrhosis) are provided in Table 1 and Table 2.

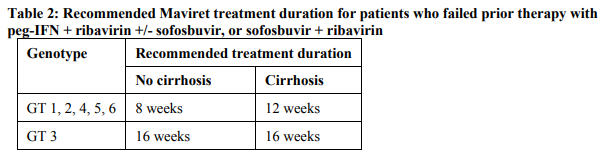
For patients who failed prior therapy with an NS3/4A- and/or an NS5A-inhibitor, see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
Patients with genotype 3 infection who are treatment-naïve without cirrhosis
A numerically lower SVR12 rate was achieved in genotype 3a-infected patients with NS5A A30K RAV at baseline treated for 8 weeks as compared to those treated for 12 weeks [84% (16/19) vs 93% (13/14)]. Relapse rates were numerically higher in patients treated for 8 weeks as compared to those treated for 12 weeks [15.8 % (3/19) vs 0 % (0/13)]. In patients without NS5A A30K RAV, there was no difference in the SVR12 rates between patients treated for 8 weeks as compared to those treated for 12 weeks [99% (189/191) vs 99% (263/266)] (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Patients with HIV-1 Co-infection
Follow the dosing recommendations in Tables 1 and 2. For dosing recommendations with HIV antiviral agents, refer to section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
Paediatric population
No dose adjustment of Maviret is required in adolescents 12 years and older (see sections 5.1 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). The safety and effectiveness of Maviret in patients less than 12 years of age have not been established.
Elderly
No dose adjustment of Maviret is required in elderly patients (see sections 5.1 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Renal impairment
No dose adjustment of Maviret is required in patients with any degree of renal impairment including patients on dialysis (see sections 5.1 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic impairment
No dose adjustment of Maviret is required in patients with mild hepatic impairment (Child-Pugh A).
Maviret is contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C) or those with any history of prior hepatic decompensation (see sections 4.3, 4.4, and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Liver or Kidney Transplant Patients
Maviret may be used for 12 weeks in liver or kidney transplant recipients. A 16-week treatment duration should be considered in transplant patients for whom a longer treatment duration is currently indicated for non-transplant patients (see sections 4.2 and 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Missed dose
In case a dose of Maviret is missed, the prescribed dose can be taken within 18 hours after the time it was supposed to be taken. If more than 18 hours have passed since Maviret is usually taken, the missed dose should not be taken and the patient should take the next dose per the usual dosing schedule. Patients should be instructed not to take a double dose.
If vomiting occurs within 3 hours of dosing, an additional dose of Maviret should be taken. If vomiting occurs more than 3 hours after dosing, an additional dose of Maviret is not needed.
Method of administration
For oral use.
Patients should be instructed to swallow tablets whole or cut in half, with food but not to chew, crush or break the tablets as it may alter the bioavailability of the agents (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).