ZEFFIX TABLETS 100 mg
Indications
Zeffix is indicated for the treatment of patients ≥ 16 years of age with chronic hepatitis B and evidence of hepatitis B virus (HBV) replication with one or more of the following conditions:
- elevated serum alanine aminotransferase (ALT) ≥ 2 times normal
- liver cirrhosis
- decompensated liver disease
- biopsy-proven necro-inflammatory liver disease
- immunocompromised state
- liver transplant
Contraindications
Zeffix is contraindicated in patients with known hypersensitivity to lamivudine or to any ingredient of the preparation.
Dosage and Administration
Pharmaceutical Form
Film-coated tablets
The recommended dosage of Zeffix is 100mg once daily.
Zeffix can be taken with or without food.
Optimum duration of therapy has not been established.
Discontinuation of Zeffix may be considered in the following situation:
- in immunocompetent patients with HBeAg and/or HBsAg seroconversion confirmed
- when a female patient becomes pregnant during therapy
- when a patient shows signs of intolerance to Zeffix while on treatment
- where in the treating doctor’s opinion, there is a loss of efficacy of Zeffix e.g. when persistent return of serum ALT to pre-treatment values or when the patient experiences deterioration in liver histology
Patient compliance should be monitored while on Zeffix therapy. If Zeffix is discontinued, patients should be periodically monitored for evidence of recurrent hepatitis (see Warnings and Precautions – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Discontinuation of treatment is not recommended in patients with decompensated liver disease. There are limited data regarding the maintenance of seroconversion long term after stopping treatment with Zeffix.
Zeffix should be used in accordance with available official recommendations.
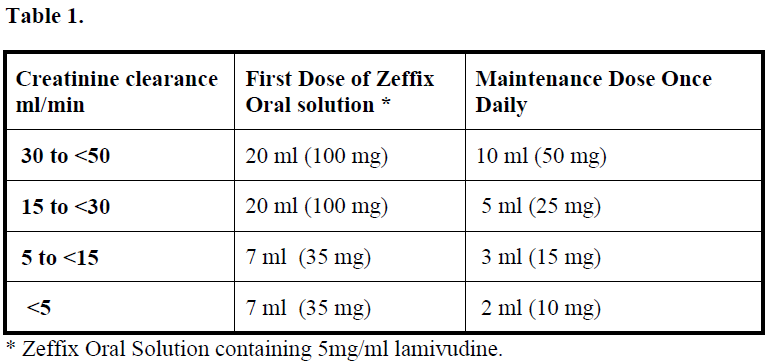
Renal impairment:
Lamivudine serum concentrations (AUC) are increased in patients with moderate to severe renal impairment due to decreased renal clearance. The dosage should therefore be reduced for patients with a creatinine clearance of < 50 ml/minute.
When doses below 100 mg are required Zeffix oral solution should be used (see table below, Table 1).
Data available in patients undergoing intermittent haemodialysis (≤ 4hrs dialysis 2–3 times weekly), indicate that following the initial dosage reduction of Zeffix to correct for the patient's creatinine clearance, no further dosage adjustments are required while undergoing dialysis.
Hepatic impairment:
Data obtained in patients with hepatic impairment, including those with end-stage liver disease awaiting transplant, show that lamivudine pharmacokinetics are not significantly affected by hepatic dysfunction. Based on these data, no dose adjustment is necessary in patients with hepatic impairment unless accompanied by renal impairment.