PADCEV® POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION 30MG/VIAL
4.1 Therapeutic indications
Padcev is indicated for the treatment of adult patients with locally advanced (LA) or metastatic urothelial cancer (mUC) who have previously received a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor, and a platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced or metastatic setting.
4.3 Contraindications
Hypersensitivity to the active substance(s) or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
4.2 Posology and method of administration
Treatment with Padcev should be initiated and supervised by a physician experienced in the use of anti-cancer therapies.
Posology
The recommended dose of Padcev is 1.25 mg/kg (up to a maximum of 125 mg for patients ≥100 kg) administered as an intravenous infusion over 30 minutes on Days 1, 8 and 15 of a 28-day cycle until disease progression or unacceptable toxicity.
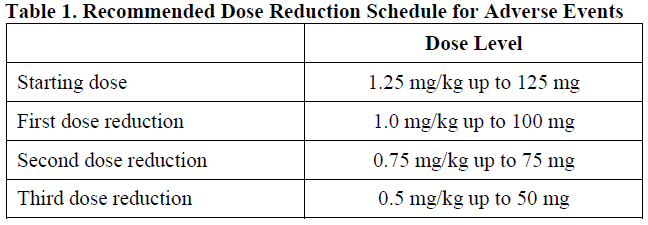
Dose Modifications
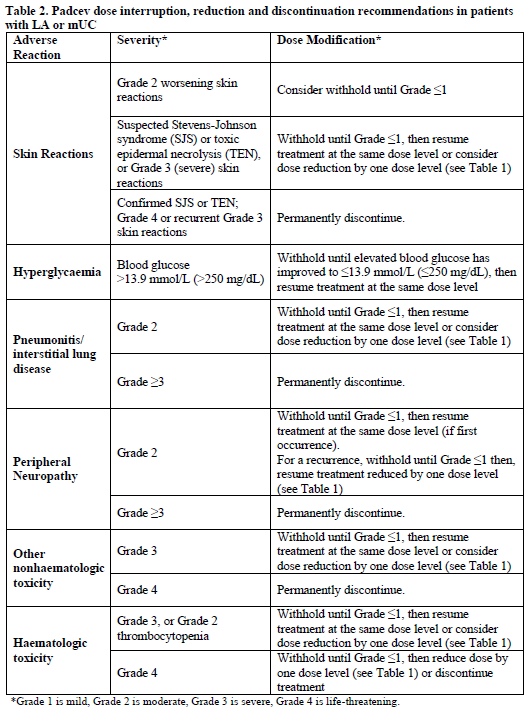
Special Populations
Elderly
No dose adjustment is necessary in patients ≥65 years of age (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Patients with Renal Impairment
No dose adjustment is necessary in patients with mild [creatinine clearance (CrCL) >60–90 mL/min], moderate (CrCL 30–60 mL/min) or severe (CrCL <30 mL/min) renal impairment (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Patients with Hepatic Impairment
No dose adjustment is necessary in patients with mild hepatic impairment (bilirubin of 1 to 1.5 × ULN and AST < ULN, or bilirubin ≤ ULN and AST > ULN). Padcev is not recommended in patients with moderate or severe hepatic impairment (AST or ALT >2.5 × ULN or total bilirubin >1.5 × ULN) as there is limited to no safety and efficacy in these patient populations (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric population
There is no relevant use of Padcev in the paediatric population for the indication of LA or mUC.
Method of administration
The recommended dose of Padcev must be administered by intravenous infusion over 30 minutes. Padcev must not be administered as an intravenous push or bolus injection.
For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.