ZERBAXA POWDER FOR SOLUTION FOR INJECTION 1G/0.5G
1. INDICATIONS AND USAGE
ZERBAXA (ceftolozane and tazobactam) for injection is indicated for the treatment of patients 18 years or older with the following infections caused by designated susceptible microorganisms.
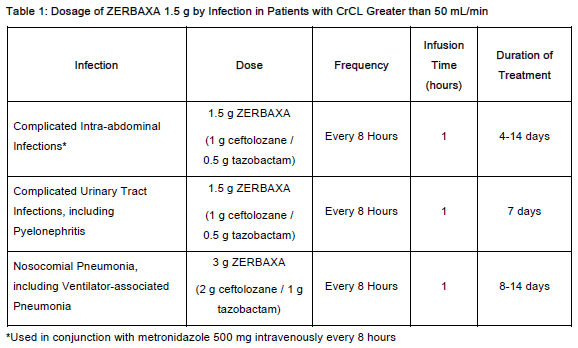
Complicated Intra-abdominal Infections
ZERBAXA used in combination with metronidazole is indicated for the treatment of complicated intra-abdominal infections (cIAI) caused by the following Gram-negative and Gram-positive microorganisms: Enterobacter cloacae, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Bacteroides fragilis, Streptococcus anginosus, Streptococcus constellatus, and Streptococcus salivarius.
Complicated Urinary Tract Infections, including Pyelonephritis
ZERBAXA is indicated for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by the following Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa.
Nosocomial Pneumonia, including Ventilator-associated Pneumonia
ZERBAXA is indicated for the treatment of nosocomial pneumonia, including ventilator-associated pneumonia (VAP), caused by the following Gram-negative microorganisms: Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella (Enterobacter) aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Serratia marcescens.
Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ZERBAXA and other antibacterial drugs, ZERBAXA should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
3. CONTRAINDICATIONS
ZERBAXA is contraindicated in patients with:
- Hypersensitivity to the active substances or to any of the inactive excipients;
- Hypersensitivity to any cephalosporin antibacterial agent;
- Severe hypersensitivity (e.g., anaphylactic reaction, severe skin reaction) to any other type of beta-lactam antibacterial agent (e.g., penicillins or carbapenems).
2. DOSAGE AND ADMINISTRATION
2.1 General
Recommended Dosage
The recommended dosage regimen of ZERBAXA for injection is 1.5 gram (g) (ceftolozane 1 g and tazobactam 0.5 g) for cIAI and cUTI and 3 g (ceftolozane 2 g and tazobactam 1 g) for nosocomial pneumonia administered every 8 hours by intravenous infusion over 1 hour in patients 18 years or older and creatinine clearance (CrCL) greater than 50 mL/min. The duration of therapy should be guided by the severity and site of infection and the patient's clinical and bacteriological progress as shown in Table 1.
Preparation of Solutions
ZERBAXA does not contain a bacteriostatic preservative. Aseptic technique must be followed in preparing the infusion solution.
Preparation of doses:
Constitute each vial of ZERBAXA with 10 mL of sterile water for injection or 0.9% Sodium Chloride for injection, USP and gently shake to dissolve. The final volume is approximately 11.4 mL per vial. CAUTION: THE CONSTITUTED SOLUTION IS NOT FOR DIRECT INJECTION.
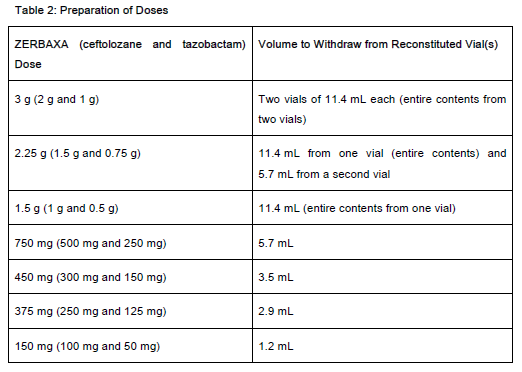
To prepare the required dose, withdraw the appropriate volume determined from Table 2 from the reconstituted vial(s). Add the withdrawn volume to an infusion bag containing 100 mL of 0.9% Sodium Chloride for Injection, USP or 5% Dextrose Injection, USP.
Inspect drug products visually for particulate matter and discoloration prior to use. ZERBAXA infusions range from clear, colorless solutions to solutions that are clear and slightly yellow. Variations in color within this range do not affect the potency of the product.
Storage of Constituted Solutions
Upon constitution with sterile water for injection or 0.9% sodium chloride injection, reconstituted ZERBAXA solution may be held for 1 hour prior to transfer and dilution in a suitable infusion bag.
Following dilution of the solution with 0.9% Sodium Chloride or 5% Dextrose, ZERBAXA is stable for 24 hours when stored at room temperature (below 25°C) or 7 days when stored under refrigeration at 2 to 8°C (36 to 46°F).
Constituted ZERBAXA solution or diluted ZERBAXA infusion should not be frozen.
Compatibility
Compatibility of ZERBAXA with other drugs has not been established. ZERBAXA should not be mixed with other drugs or physically added to solutions containing other drugs.
2.2 Renal Impairment
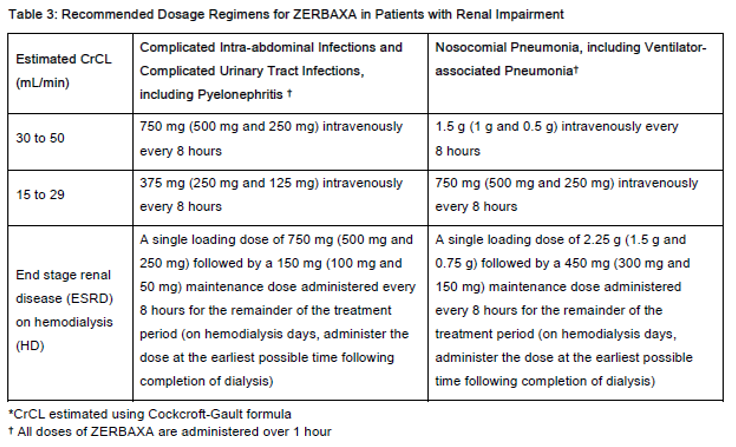
Dose adjustment is required for patients whose CrCL is 50 mL/min or less. Renal dose adjustments are listed in Table 3. For patients with changing renal function, monitor CrCL at least daily and adjust the dosage of ZERBAXA accordingly [see Warnings and Precautions (4.1) and Use in Special Populations (6.5) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information].
2.3 Hepatic impairment
No dose adjustment is necessary in patients with hepatic impairment.