SONAZOID POWDER AND SOLVENT FOR DISPERSION FOR INJECTION 16 MICROLITRE PER VIAL
Therapeutic indications
This medicinal product is for diagnostic use only.
Sonazoid is an ultrasound contrast agent for use in vascular phase and Kupffer phase for ultrasonic imaging of focal hepatic lesions.
Contraindications
Hypersensitivity to the active substance or to any of the excipients.
Posology and method of administration
This medicinal product should always be given by a physician or other qualified health personnel.
The usual adult dosage is up to one vial of the product containing 16 microlitre of perfluorobutane (PFB) microbubbles (MB) suspended in 2 ml of the accompanying sterile water for reconstitution to make an 8 microlitre/ml suspension. The product is for intravenous use only and the usual dosage is as per the table below.
Before administering Sonazoid, see Special precautions for disposal and other handling for instructions for reconstitution and use – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
The reconstituted product is for intravenous use. No special preparation of the patient is required. Ultrasound imaging must be performed during injection of Sonazoid as optimal contrast effect is obtained immediately after administration. The intravenous line must be flushed immediately with 5–10 ml sodium chloride 0.9% solution for injection to ensure complete administration of the contrast agent.
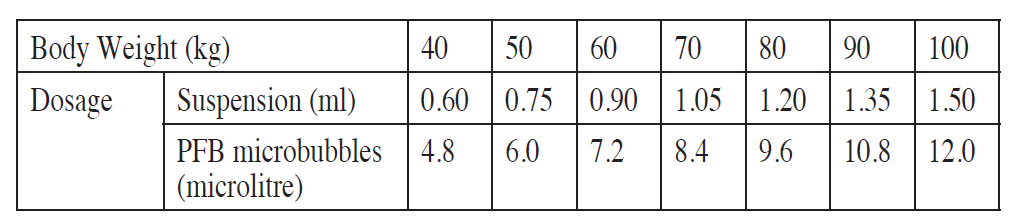
The recommended clinical dose is 0.12 microlitre PFB microbubbles/kg body weight (is equivalent to 0.015 ml/kg as a suspension).
Refer to the table below for weight-based dosages.
Use in elderly
The usual/proposed dose for adults can be used.
Paediatric use
The safety of this product has not been established in the paediatric population (no data are available).