RotaTeq
III. INDICATIONS
RotaTeq is an oral pentavalent vaccine indicated for the prevention of severe rotavirus gastroenteritis in infants and children (See II. CLINICAL PHARMACOLOGY section – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
V. CONTRAINDICATIONS
The administration of RotaTeq should be postponed in subjects suffering from acute diarrhea or vomiting.
Hypersensitivity to any component of the vaccine.
Individuals who develop symptoms suggestive of hypersensitivity after receiving a dose of RotaTeq should not receive further doses of RotaTeq.
Subjects with congenital malformation of the gastrointestinal tract.
Infants who have known or suspected immunodeficiency. Asymptomatic HIV infection is not expected to affect the safety or efficacy of RotaTeq. However, in the absence of sufficient data, administration of RotaTeq to asymptomatic HIV subjects is not recommended.
Individuals with Severe Combined Immunodeficiency Disease (SCID). Cases of gastroenteritis associated with vaccine virus have been reported post-marketing in infants with SCID.
Previous history of intussusception.
IV. DOSAGE AND ADMINISTRATION
FOR ORAL USE ONLY. NOT FOR INJECTION.
Posology
The vaccination series consists of three ready-to-use liquid doses of RotaTeq administered orally to infants.
The first dose of RotaTeq should be administered at 6 to 12 weeks of age; the subsequent doses should be administered at a minimum interval of 4 weeks between each dose. The third dose should not be administered after 32 weeks of age (See IX. PEDIATRIC USE – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
There are no restrictions on the infant’s consumption of food or liquid, including breast milk, either before or after vaccination with RotaTeq.
RotaTeq may be given to pre-term infants according to their chronological age.
If for any reason an incomplete dose is administered (e.g., infant spits or regurgitates the vaccine), a replacement dose is not recommended, since such dosing was not studied in the clinical trials. The infant should continue to receive any remaining doses in the recommended series.
The vaccine is to be administered orally without mixing with any other vaccines or solutions. Do not reconstitute or dilute.
Each dose is supplied in a container consisting of a squeezable plastic, latex-free dosing tube with a twist-off cap, allowing for direct oral administration. The dosing tube is contained in a pouch.
To administer the vaccine:
Tear open the pouch and remove the dosing tube.
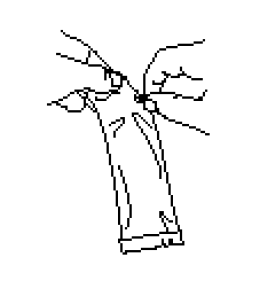
Clear the fluid from the dispensing tip by holding tube vertically and tapping cap.
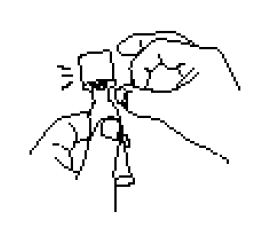
Open the dosing tube in 2 easy motions:
Puncture the dispensing tip by screwing cap clockwise until it becomes tight.
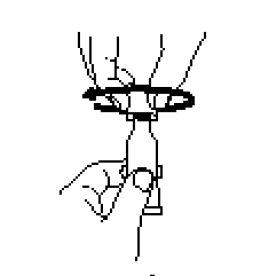
Remove cap by turning it counterclockwise.
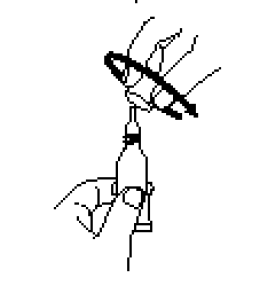
Administer dose by gently squeezing liquid into infant’s mouth toward the inner cheek until dosing tube is empty. (A residual drop may remain in the tip of the tube.)
Discard the empty tube and cap in approved biological waste containers according to local regulations.
Use with Other Vaccines
RotaTeq can be administered with diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine, inactivated or oral poliovirus vaccine (IPV or OPV), Haemophilus influenzae type b conjugate vaccine, hepatitis B vaccine, pneumococcal conjugate vaccine, meningococcal group C conjugate vaccine, and hexavalent vaccines.
Concomitant administration of RotaTeq and oral poliovirus vaccine (OPV) does not affect the immune response to the poliovirus antigens, but may reduce that to RotaTeq. The immune responses to RotaTeq are unaffected when OPV is administered two weeks after RotaTeq.