LONSURF FILM-COATED TABLET 20MG/8.19MG
1 INDICATIONS AND USAGE
1.1 Metastatic Colorectal Cancer
Lonsurf® is indicated for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy.
1.2 Metastatic Gastric Cancer
Lonsurf® is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma who have been previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neutargeted therapy.
4 CONTRAINDICATIONS
None.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
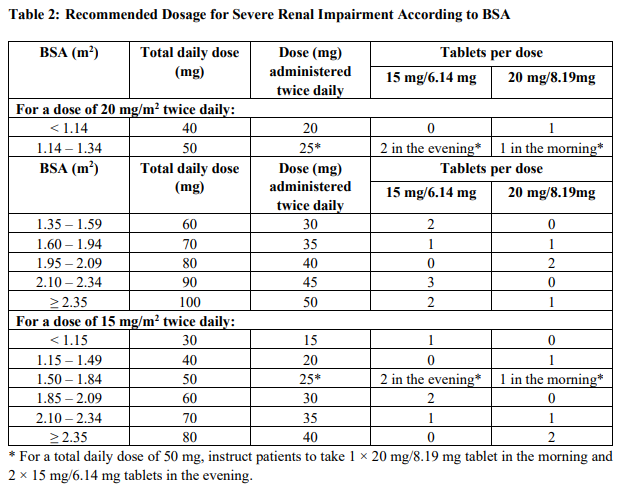
The recommended dosage of Lonsurf® is 35 mg/m2 up to a maximum of 80 mg per dose (based on the trifluridine component) orally twice daily within one hour of completion of morning and evening meals on Days 1 through 5 and Days 8 through 12 of each 28-day cycle until disease progression or unacceptable toxicity. Round dose to the nearest 5 mg increment.
Instruct patients to swallow Lonsurf® tablets whole.
Instruct patients not to retake doses of Lonsurf® that are vomited or missed and to continue with the next scheduled dose.
Table 1 shows the calculated initial daily dose based on body surface area (BSA).
2.2 Dosage Modifications for Adverse Reactions
Obtain complete blood cell counts prior to and on Day 15 of each cycle. [see Warnings and Precautions (5.1) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ]
Do not initiate the cycle of Lonsurf® until:
- Absolute neutrophil count (ANC) is greater than or equal to 1,500/mm3 or febrile neutropenia is resolved
- Platelets are greater than or equal to 75,000/mm3
- Grade 3 or 4 non-hematological adverse reactions are resolved to Grade 0 or 1
Within a treatment cycle, withhold Lonsurf® for any of the following:
- Absolute neutrophil count (ANC) less than 500/mm3 or febrile neutropenia
- Platelets less than 50,000/mm3
- Grade 3 or 4 non-hematological adverse reaction
After recovery, resume Lonsurf® after reducing the dose by 5 mg/m2/dose from the previous dose, if the following occur:
- Febrile neutropenia
- Uncomplicated Grade 4 neutropenia (which has recovered to greater than or equal to 1,500/mm3) or thrombocytopenia (which has recovered to greater than or equal to 75,000/mm3) that results in more than 1 week delay in start of next cycle
- Non-hematologic Grade 3 or Grade 4 adverse reaction except for Grade 3 nausea and/or vomiting controlled by antiemetic therapy or Grade 3 diarrhea responsive to antidiarrheal medication
A maximum of 3 dose reductions are permitted. Permanently discontinue Lonsurf® in patients who are unable to tolerate a dose of 20 mg/m2 orally twice daily. Do not escalate Lonsurf® dosage after it has been reduced.
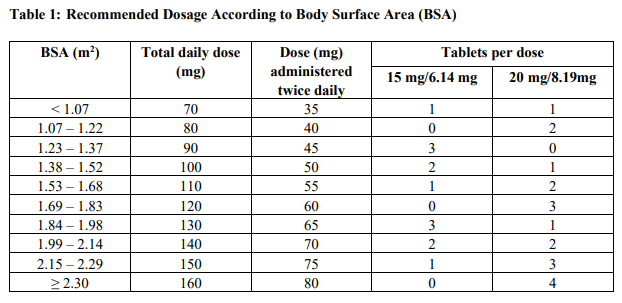
2.3 Recommended Dosage for Renal Impairment
Severe Renal Impairment
In patients with severe renal impairment [creatinine clearance (CLcr) of 15 to 29 mL/min as determined by the Cockcroft-Gault formula], the recommended initial dosage is 20 mg/m2 (based on the trifluridine component) orally twice daily within one hour of completion of morning and evening meals on Days 1 through 5 and Days 8 through 12 of each 28-day cycle (Table 2). [see Use in Specific Populations (7.7), Clinical Pharmacology (10.3) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ] Reduce dose to 15 mg/m2 twice daily in patients with severe renal impairment who are unable to tolerate a dose of 20 mg/m2 twice daily (Table 2). Permanently discontinue Lonsurf® in patients who are unable to tolerate a dose of 15 mg/m2 twice daily.