ALOXI® Solution for Injection 50mcg/ml
INDICATIONS AND USAGE
Chemotherapy-Induced Nausea and Vomiting in Adults
Aloxi ® is indicated for:
- Moderately emetogenic cancer chemotherapy -- prevention of acute and delayed nausea and vomiting associated with initial and repeat courses
- Highly emetogenic cancer chemotherapy -- prevention of acute nausea and vomiting associated with initial and repeat courses
Chemotherapy-Induced Nausea and Vomiting in Pediatric Patients Aged 1 month to Less than 17 Years
Aloxi ® is indicated for:
- Prevention of acute nausea and vomiting associated with initial and repeat courses of moderately or highly emetogenic cancer chemotherapy
Postoperative Nausea and Vomiting in Adults
Aloxi ® is indicated for:
- Prevention of postoperative nausea and vomiting (PONV) for up to 24 hours following surgery. Efficacy beyond 24 hours has not been demonstrated.
As with other antiemetics, routine prophylaxis is not recommended in patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and vomiting must be avoided during the postoperative period, Aloxi ® is recommended even where the incidence of postoperative nausea and/or vomiting is low.
CONTRAINDICATIONS
Aloxi ® is contraindicated in patients known to have hypersensitivity to the drug or any of its components. [ see Adverse Reactions – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information]
DOSAGE AND ADMINISTRATION
Recommended Dosing
Chemotherapy-Induced Nausea and Vomiting
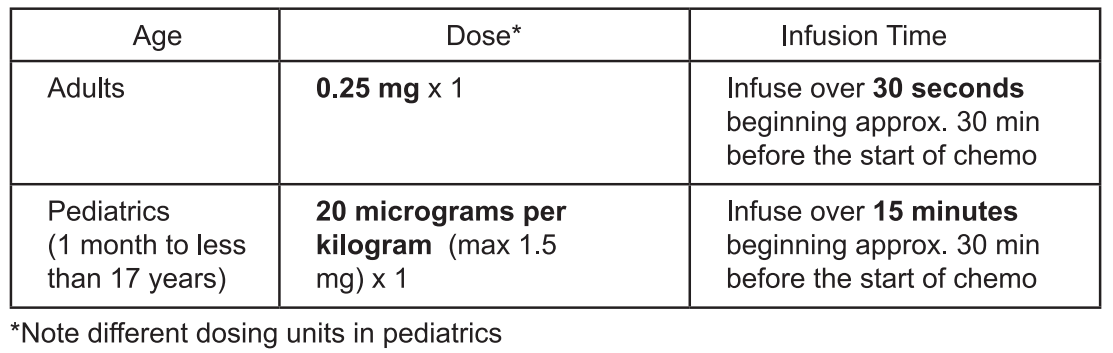
The safety and efficacy of Aloxi in children aged less than 1 month have not been established. No data are available. There is limited data on the use of Aloxi in the prevention of nausea and vomiting in children under 2 years of age.
Postoperative Nausea and Vomiting
Dosage for Adults – a single 0.075 mg I.V. dose administered over 10 seconds immediately before the induction of anesthesia.
Instructions for I.V. Administration
Aloxi ® is supplied ready for intravenous administration at a concentration of 0.05 mg/mL (50 mcg/mL). Aloxi ® should not be mixed with other drugs. Flush the infusion line with normal saline before and after administration of Aloxi ®.
Parenteral drug products should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit.