IMFINZI CONCENTRATE FOR SOLUTION FOR INFUSION 50 MG/ML
4.1 Therapeutic indications
Locally Advanced Non-small Cell Lung Cancer (NSCLC)
IMFINZI is indicated for the treatment of patients with locally advanced, unresectable NSCLC whose disease has not progressed following platinum-based chemoradiation therapy.
Small Cell Lung Cancer (SCLC)
IMFINZI in combination with etoposide and either carboplatin or cisplatin is indicated for the first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC).
Biliary Tract Cancer (BTC)
IMFINZI in combination with cisplatin and gemcitabine, is indicated for the treatment of patients with locally advanced or metastatic biliary tract cancer (BTC).
Hepatocellular Carcinoma (HCC)
IMFINZI in combination with tremelimumab is indicated for the treatment of patients with unresectable hepatocellular carcinoma (uHCC) who have not received prior systemic therapy.
4.3 Contraindications
Hypersensitivity to the active ingredient or to any of the excipients.
4.2 Posology and method of administration
The recommended dose of IMFINZI depends on the indication as presented in Table 1. IMFINZI is administered as an intravenous infusion over 1 hour.
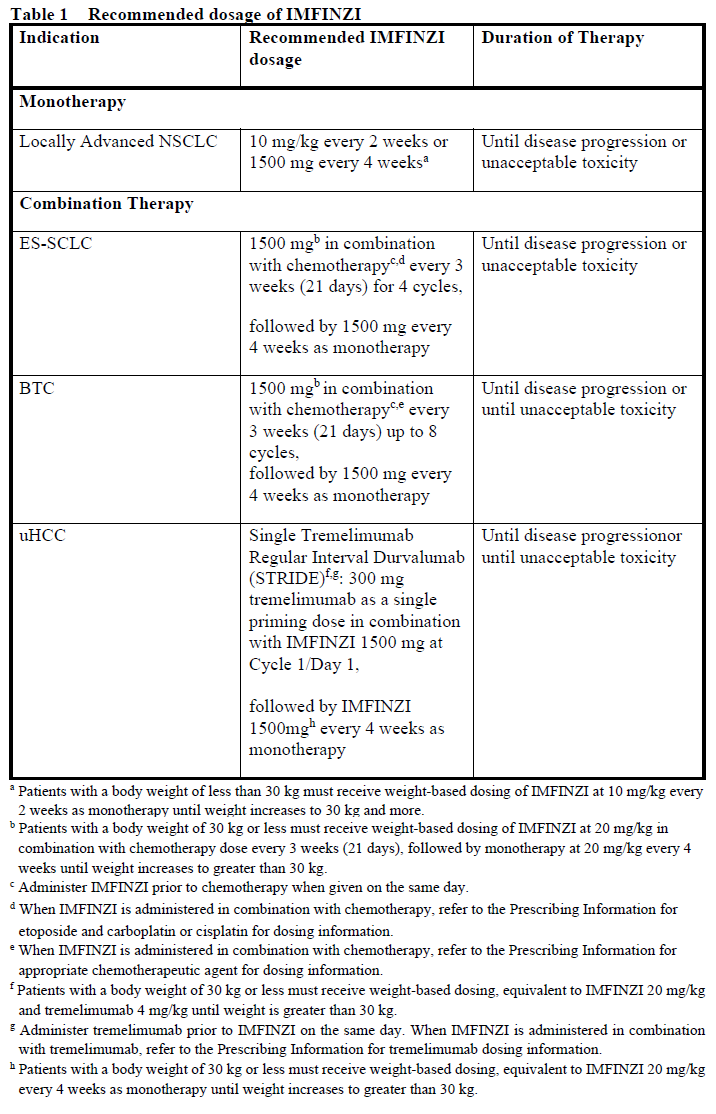
No dose reduction or escalation for IMFINZI is recommended. In general, withhold IMFINZI for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue IMFINZI for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids.
Immune-mediated adverse reactions requiring specific management are summarized in Table 2. Refer to section 4.4 for further monitoring and evaluation information – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
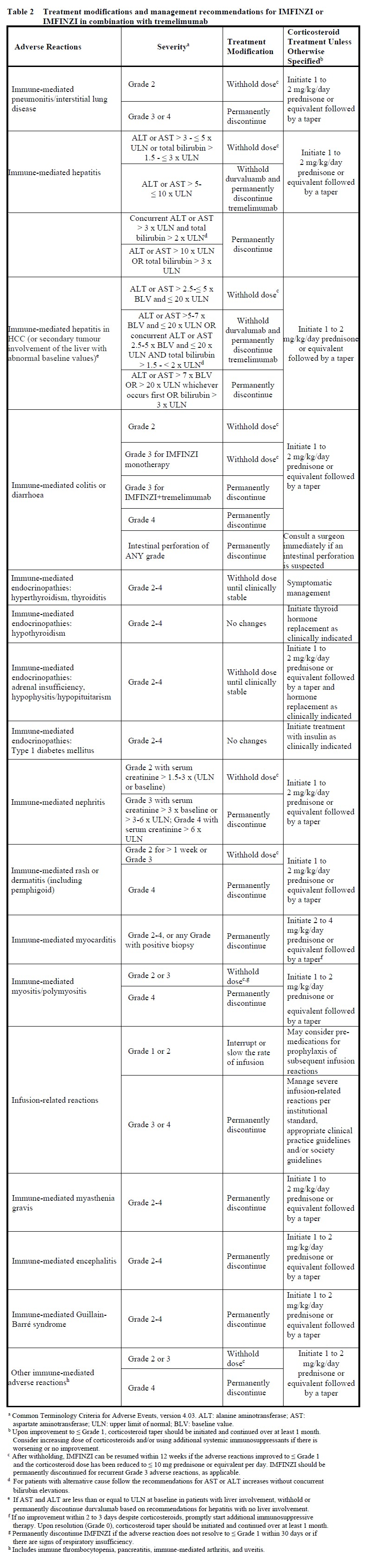
For suspected immune-mediated adverse reactions, adequate evaluation should be performed to confirm etiology or exclude alternate etiologies.
For non-immune-mediated adverse reactions, withhold IMFINZI for Grade 2 and 3 adverse reactions until ≤ Grade 1 or baseline. IMFINZI should be discontinued for Grade 4 adverse reactions (with the exception of Grade 4 laboratory abnormalities, about which the decision to discontinue should be based on accompanying clinical signs/symptoms and clinical judgment).
Special patient populations
Based on a population pharmacokinetic analysis, no dose adjustment of IMFINZI is recommended based on patient age, body weight, gender and race (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric and adolescents
The safety and effectiveness of IMFINZI have not been established in children and adolescents aged less than 18 years.
Elderly (≥ 65 years)
No dose adjustment is required for elderly patients (≥ 65 years of age) (see sections 5.1 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Renal impairment
No dose adjustment of IMFINZI is recommended in patients with mild or moderate renal impairment. Data from patients with severe renal impairment are too limited to draw conclusions on this population (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic impairment
Based on a population pharmacokinetic analysis, no dose adjustment of IMFINZI is recommended for patients with mild or moderate hepatic impairment. IMFINZI has not been studied in patients with severe hepatic impairment (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Method of administration
For intravenous administration.
For instructions on dilution of the medicinal product before administration, see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.