COMIRNATY, Dispersion for Injection, 30 micrograms/dose
4.1 Therapeutic indications
COMIRNATY is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 virus, in individuals 6 months of age and older.
The use of this vaccine should be in accordance with official recommendations.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
4.2 Posology and method of administration
Posology
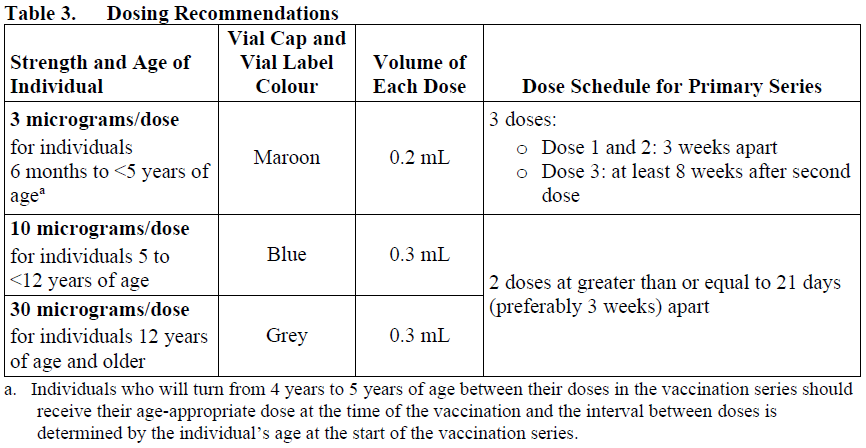
A booster may be administered in accordance with official recommendations.
Individuals may not be protected until at least 7 days after their second dose of the vaccine (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Additional booster doses in individuals 12 years of age and older
Any subsequent doses of COMIRNATY may be administered at least 3 months after a previous dose of COMIRNATY and in accordance with official recommendations.
Interchangeability with other COVID-19 vaccines
The interchangeability of COMIRNATY with other COVID-19 vaccines has not been established.
Interchangeability of COMIRNATY (Original) with variant-adapted presentations of COMIRNATY
The primary series and booster may consist of either COMIRNATY (Original), or a variant adapted presentation of COMIRNATY, or a combination, but not exceeding the total number of doses recommended for the primary series. The primary series should only be administered once.
Paediatric population
The safety and efficacy of COMIRNATY in paediatric participants aged less than 6 months have not yet been established. Limited data are available.
Elderly population
No dosage adjustment is required in elderly individuals ≥65 years of age. The safety of a booster dose of COMIRNATY (Original) in individuals 65 years of age and older is based on safety data in 12 booster dose recipients 65 through 85 years of age in Study 2, 306 booster dose recipients 18 through 55 years of age in Study 2, and 1,175 booster dose recipients 65 years of age and older in Study 4. The effectiveness of a booster dose of COMIRNATY in individuals 65 years of age and older is based on effectiveness data in 306 booster dose recipients 18 through 55 years of age in Study 2, and an efficacy analysis from participants 16 years of age and older in 9,945 participants in Study 4.
Method of administration
Administer COMIRNATY intramuscularly. Do not administer intravascularly, subcutaneously, or intradermally.
- In individuals 6 months to <12 months of age: administer COMIRNATY in the anterolateral aspect of the thigh.
- In individuals 1 year to <5 years of age: administer COMIRNATY in the anterolateral aspect of the thigh or the deltoid muscle.
- In individuals 5 years of age and older: administer COMIRNATY in the deltoid muscle.
For detailed instructions on the handling, dilution, and dose preparation of the vaccine before administration, see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.