JARDIANCE FILM-COATED TABLET 25MG
4.1 Therapeutic indications
Type 2 diabetes mellitus
JARDIANCE is indicated in the treatment of type 2 diabetes mellitus to improve glycaemic control in adults as:
Monotherapy
When diet and exercise alone do not provide adequate glycaemic control in patients for whom use of metformin is considered inappropriate due to intolerance.
Add-on combination therapy
In combination with other glucose-lowering medicinal products including insulin, when these, together with diet and exercise, do not provide adequate glycaemic control (see sections 4.4, 4.5 and 5.1 for available data on different combinations – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Add-on combination in patients with established cardiovascular disease
JARDIANCE is indicated as an adjunct to diet, exercise and standard care therapy to reduce the incidence of cardiovascular death in patients with type 2 diabetes mellitus and established cardiovascular disease who have inadequate glycemic control (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Heart failure (HF)
JARDIANCE is indicated in adult patients with heart failure (NYHA class II–IV), with or without type 2 diabetes mellitus to reduce the risk of cardiovascular death and hospitalization for heart failure (see clinical trials – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
4.2 Posology and method of administration
Posology
Type 2 diabetes mellitus
Monotherapy and add-on combination
The recommended starting dose is 10 mg empagliflozin once daily for monotherapy and add-on combination therapy with other medicinal products including insulin. In patients tolerating empagliflozin 10 mg once daily and need tighter glycaemic control, the dose can be increased to 25 mg once daily. The maximum daily dose is 25 mg (see below and section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
When empagliflozin is used in combination with a sulphonylurea or with insulin, a lower dose of the sulphonylurea or insulin may be considered to reduce the risk of hypoglycaemia (see sections 4.5 and 4.8 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Heart failure (HF)
The recommended dose of JARDIANCE is 10 mg once daily (see clinical trial section – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Special populations
Renal impairment
Dose adjustment recommendations*
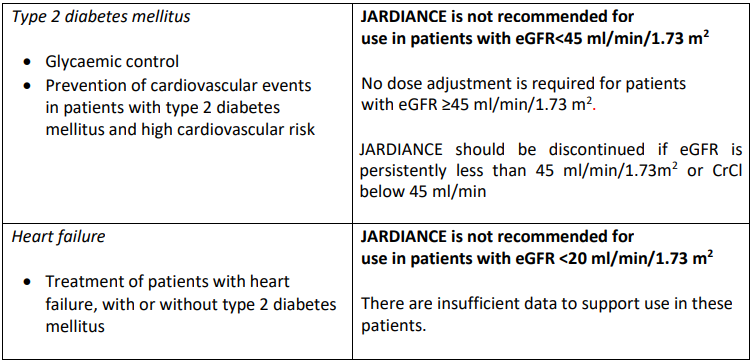
*see section 4.4, 4.8, 5.1 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information
Empagliflozin should not be used in patients with end stage renal disease (ESRD) or in patients on dialysis. There are insufficient data to support use in these patients (see section 4.4, 5.1 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic impairment
No dose adjustment is required for patients with hepatic impairment. Empagliflozin exposure is increased in patients with severe hepatic impairment. Therapeutic experience in patients with severe hepatic impairment is limited and therefore not recommended for use in this population (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Elderly patients
No dose adjustment is recommended based on age. In patients 75 years and older, an increased risk for volume depletion should be taken into account (see sections 4.4 and 4.8 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric population
The safety and efficacy of empagliflozin in children and adolescents has not yet been established. No data are available.
Method of administration
The tablets can be taken with or without food, swallowed whole with water. If a dose is missed, it should be taken as soon as the patient remembers. A double dose should not be taken on the same day.