OMNIPAQUE INJECTION 300 mg I/ml
4.1 THERAPEUTIC INDICATIONS
This medicinal product is for diagnostic use only.
X-ray contrast medium for use in adults and children for cardioangiography, arteriography, urography, phlebography and CT-enhancement. Lumbar, thoracic, cervical myelography and computed tomography of the basal cisterns, following subarachnoid injection. Arthrography, endoscopic retrograde pancreatography (ERP), endoscopic retrograde cholangiopancreatography (ERCP), herniography, hysterosalpingography, sialography and studies of the gastrointestinal tract.
4.3 CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients (See Section 6.1 LIST OF EXCIPIENTS – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information)
Manifest thyrotoxicosis.
4.2 POSOLOGY AND METHOD OF ADMINISTRATION
The dosage varies depending on the type of examination, age, weight, cardiac output and general condition of the patient and the technique used. Usually the same iodine concentration and volume is used as with other iodinated X-ray contrast media in current use. Adequate hydration should be assured before and after administration as for other contrast media.
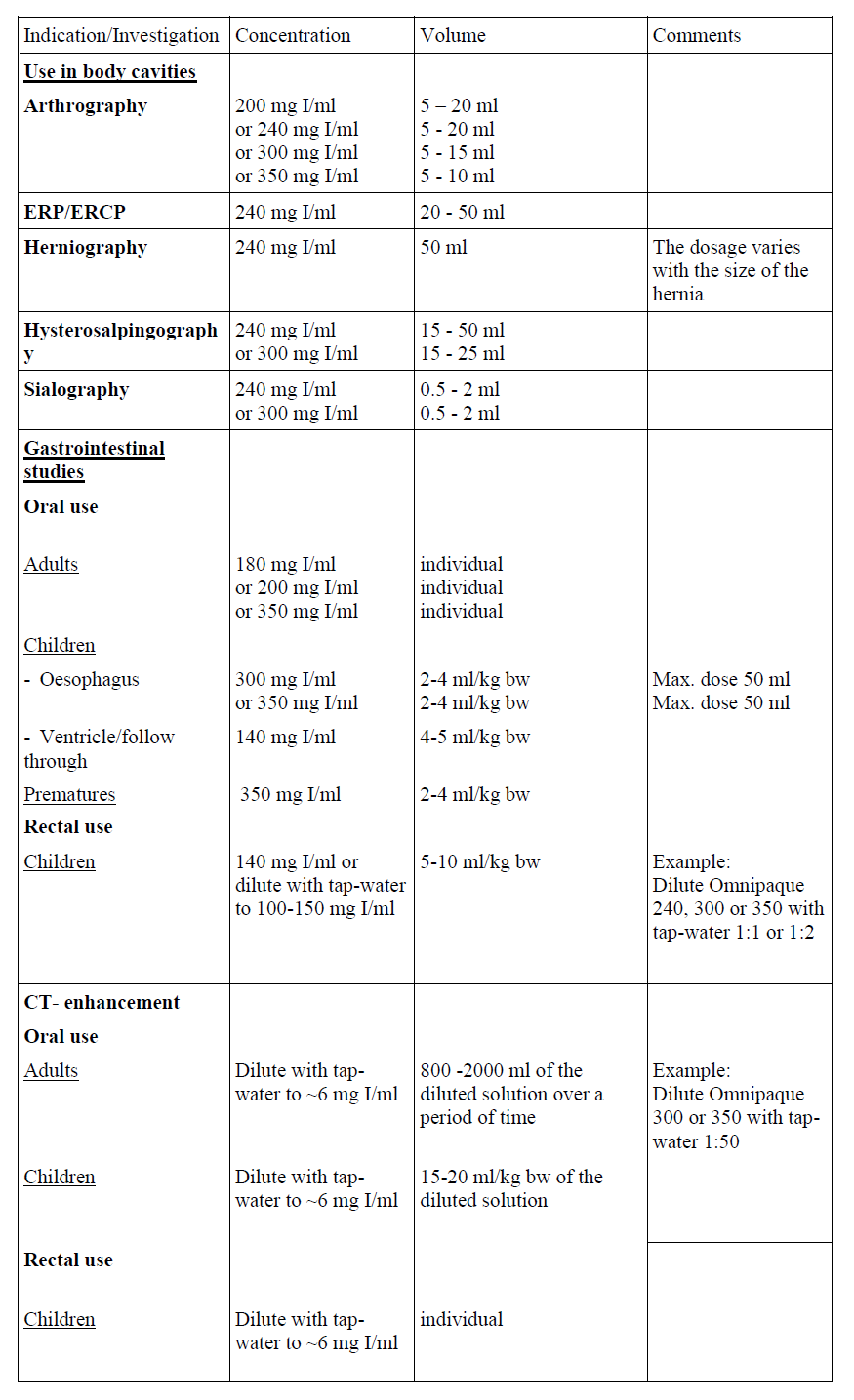
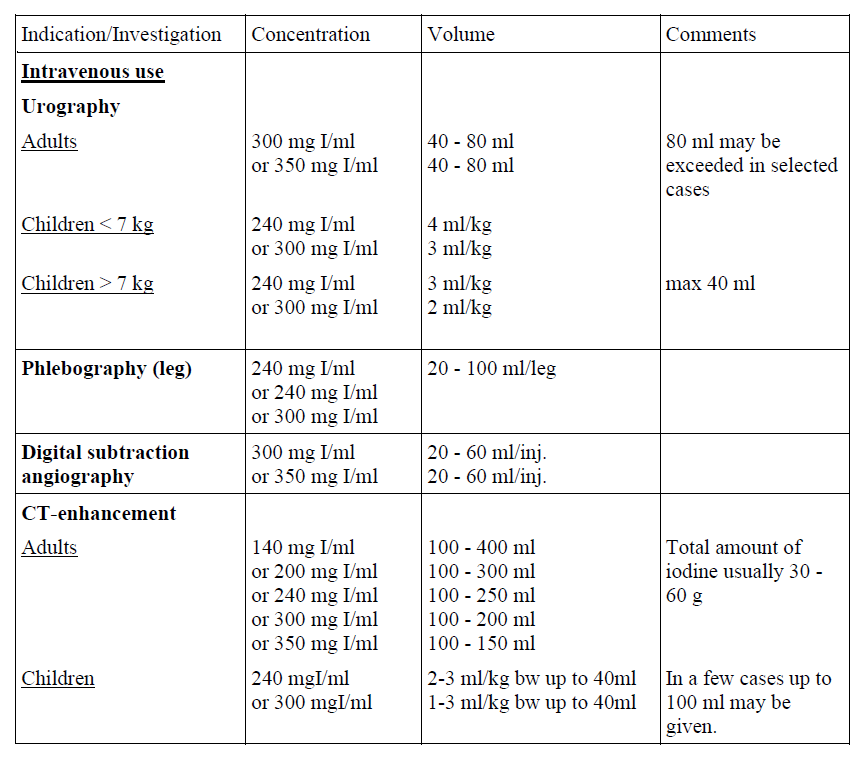
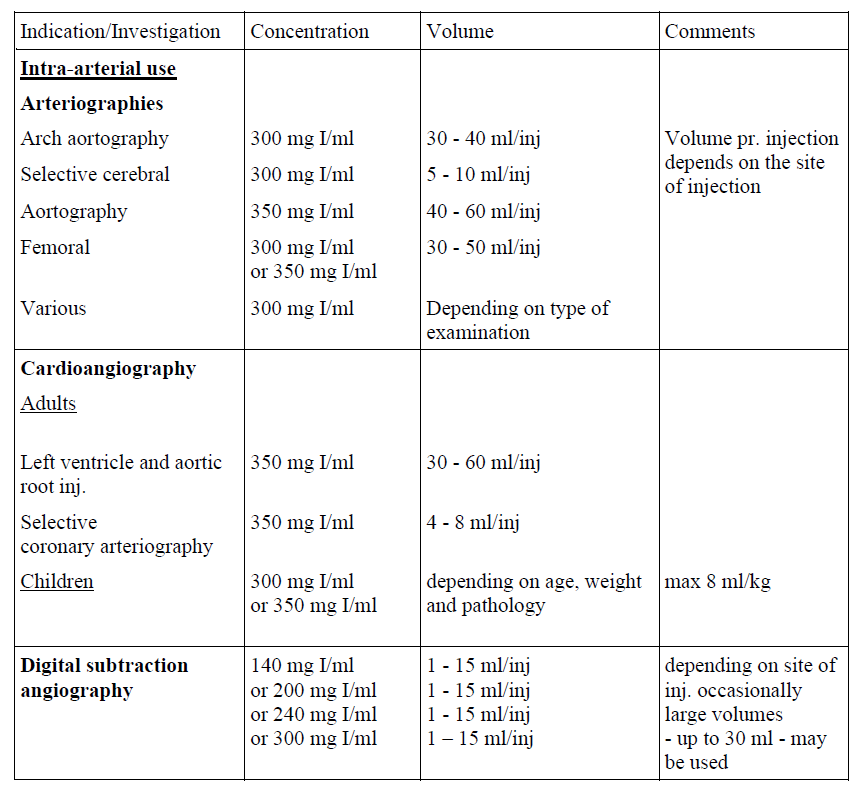
For intravenous, intra-arterial and intrathecal use, and use in body cavities.
The following dosages may serve as a guide.
Guidelines for intravenous use
Guidelines for intra-arterial use
Guidelines for intrathecal use
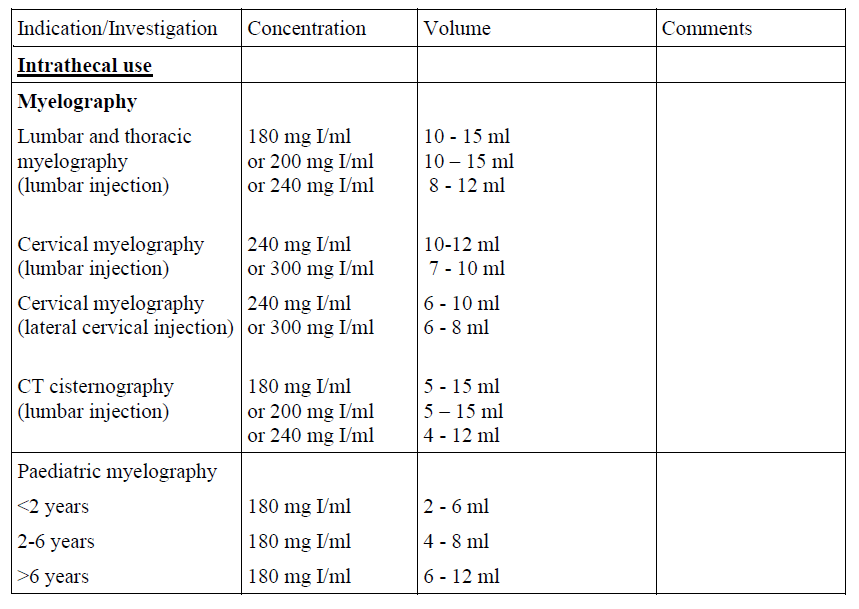
To minimize possible adverse reactions a total dose of 3 g iodine should not be exceeded.
Guidelines for Body cavities