KLACID GRANULES FOR ORAL SUSPENSION 250 mg/5 ml
4.1 Therapeutic Indications
Klacid® Pediatric Suspension is indicated for treatment of infections due to susceptible organisms in children 6 months to 12 years, in the following conditions:
- Upper respiratory infections (e.g., streptococcal pharyngitis).
- Lower respiratory infections (e.g., bronchitis, pneumonia) (see section 4.4 and 5.1 regarding Sensitivity Testing – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information)
- Acute otitis media.
- Skin and skin structure infections (e.g., impetigo, folliculitis, cellulitis, abscesses) (see section 4.4 and 5.1 regarding Sensitivity Testing – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information)
- Disseminated or localized mycobacterial infections due to Mycobacterium avium or Mycobacterium intracellulare. Localized infections due to Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium kansasii.
Consideration should be given to national official guidance on the appropriate use of antibacterial agents.
4.3. Contraindications
Hypersensitivity to macrolide antibiotic drugs or any of its excipients (see section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Concomitant administration of clarithromycin and any of the following drugs is contraindicated: astemizole, cisapride, pimozide, terfenadine as this may result in QT prolongation and cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation, and torsades de pointes (see section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Concomitant administration of clarithromycin and ergot alkaloids (e.g., ergotamine or dihydroergotamine) is contraindicated, as this may result in ergot toxicity (see section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Concomitant administration of clarithromycin and oral midazolam is contraindicated (see section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Clarithromycin should not be given to patients with history of QT prolongation (congenital or documented acquired QT prolongation) or ventricular cardiac arrhythmia, including torsades de pointes (see sections 4.4 and 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Clarithromycin should not be given to patients with electrolyte disturbances (hypokalemia or hypomagnesaemia, due to the risk of prolongation of the QT-interval).
Clarithromycin should not be used in patients who suffer from severe hepatic failure in combination with renal impairment.
Clarithromycin should not be used concomitantly with HMG-CoA reductase inhibitors (statins) that are extensively metabolized by CYP3A4 (lovastatin or simvastatin), due to the increased risk of myopathy, including rhabdomyolysis (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Clarithromycin (and other strong CYP3A4 inhibitors) should not be used concomitantly with colchicine (see sections 4.4 and 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Concomitant administration with ticagrelor or ranolazine is contraindicated.
Concomitant administration of clarithromycin and lomitapide is contraindicated (see section 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
As the dose cannot be reduced from 500 mg once-daily, clarithromycin modified release is contraindicated in patients with creatinine clearance less than 30 mL/min. Clarithromycin immediate release tablets may be utilized in this patient population.
4.2 Posology and method of administration
DOSAGE AND ADMINISTRATION
Pediatric Patients 6 months to 12 years of age
Clinical trials have been conducted using Klacid® pediatric suspension in children 6 months to 12 years of age. Therefore, children 6 months to 12 years of age should use Klacid® pediatric suspension (granules for oral suspension).
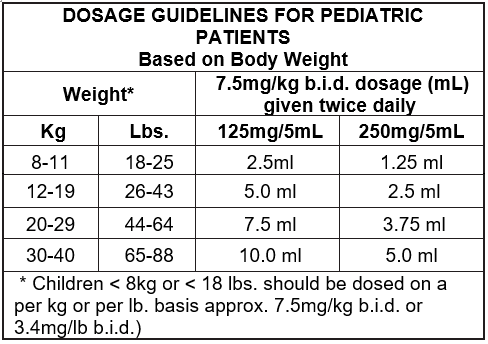
The recommended daily dosage of Klacid® Pediatric Suspension (125mg/5mL or 250mg/5mL) in children is 7.5mg/ kg b.i.d. up to a maximum dose of 500mg b.i.d. for non-mycobacterial infections. The usual duration of treatment is for five to ten days depending on the pathogen involved and the severity of the condition. The prepared suspension can be taken with or without meals, and can be taken with milk.
The following table is a suggested guide for determining dosage:
Dosage in Patients with Mycobacterial Infections
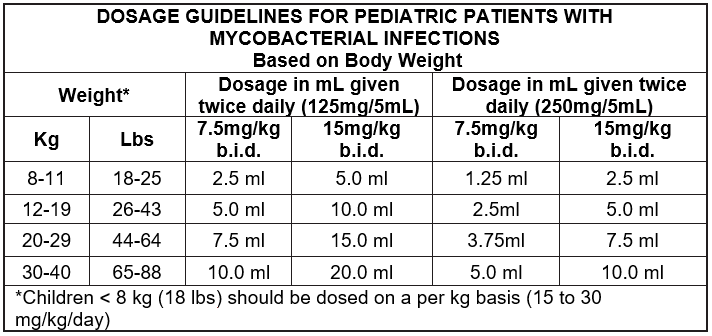
In children with disseminated or localized mycobacterial infections ( M. avium, M. intracellulare, M. chelonae, M. fortuitum, M. kansasii), the recommended dose is 15 to 30mg/kg clarithromycin b.i.d. not exceeding a maximum dose of 500 mg b.i.d.
Treatment with clarithromycin should continue as long as clinical benefit is demonstrated. The addition of other antimycobacterial agents may be of benefit.
Preparations for use
See section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information
Renal Impairment
In children with creatinine clearance less than 30 ml/min/1.73m2, the dosage of clarithromycin should be reduced by one half, i.e. up to 250mg once daily, or 250mg twice daily in more severe infections. Dosage should not be continued beyond 14 days in these patients.