TRECONDI POWDER FOR SOLUTION FOR INFUSION 1 G PER VIAL
4.1 Therapeutic indications
Treosulfan in combination with fludarabine is indicated as part of conditioning treatment prior to allogeneic haematopoietic stem cell transplantation (alloHSCT) in adult patients with acute myeloid leukaemia (AML) or myelodysplastic syndrome (MDS) at increased risk of toxicity with standard conditioning therapies, and in paediatric patients older than one month with malignant diseases.
4.3 Contraindications
- Hypersensitivity to the active substance
- Active non-controlled infectious disease
- Severe concomitant cardiac, lung, liver, and renal impairment
- Fanconi anaemia and other DNA breakage repair disorders
- Pregnancy (see section 4.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information)
- Administration of live vaccine
4.2 Posology and method of administration
Administration of treosulfan should be supervised by a physician experienced in conditioning treatment followed by alloHSCT.
Posology
Adults with AML and MDS
Treosulfan is given in combination with fludarabine.
The recommended dose and schedule of administration is:
- Treosulfan 10 g/m² body surface area (BSA) per day as a two-hour intravenous infusion, given on three consecutive days (day -4, -3, -2) before stem cell infusion (day 0). The total treosulfan dose is 30 g/m²;
- Fludarabine 30 mg/m² BSA per day as a 0.5-hour intravenous infusion, given on five consecutive days (day -6, -5, -4, -3, -2) before stem cell infusion (day 0). The total fludarabine dose is 150 mg/m²;
- Treosulfan should be administered before fludarabine on days -4, -3, -2 (FT10 regimen).
Elderly
No dose adjustment is necessary in any subset of the elderly population.
Renal and hepatic impairment
No dose adjustment is necessary for mild or moderate impairment, but treosulfan is contraindicated in patients with severe impairment (see section 4.3).
Paediatric population
Treosulfan is given in combination with fludarabine, with thiotepa (intensified regimen; FT10–14TT regimen) or without thiotepa (FT10–14 regimen).
The recommended dose and schedule of administration is:
Treosulfan 10–14 g/m² body surface area (BSA) per day as a two-hour intravenous infusion, given on three consecutive days (day -6, -5, -4) before stem cell infusion (day 0). The total treosulfan dose is 30–42 g/m²;
The dose of treosulfan should be adapted to the patient’s BSA as follows (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information):
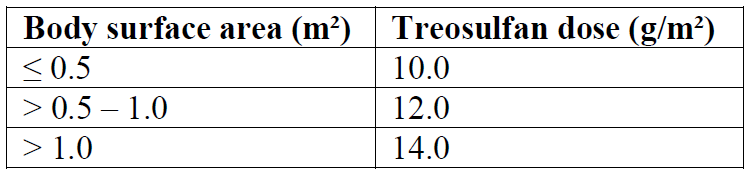
Fludarabine 30 mg/m² BSA per day as a 0.5-hour intravenous infusion, given on five consecutive days (day -7, -6, -5, -4, -3) before stem cell infusion (day 0). The total fludarabine dose is 150 mg/m²;
Treosulfan should be administered before fludarabine;
Thiotepa (intensified regimen 5 mg/kg twice a day), given as two intravenous infusions over 2– 4 hours on day -2 before stem cell infusion (day 0).
The safety and efficacy of treosulfan in children less than 1 month of age has not yet been established.
Method of administration
Treosulfan is for intravenous use as a two-hour infusion.
Precautions to be taken before handling or administering the medicinal product
When handling treosulfan, inhalation, skin contact or contact with mucous membranes should be avoided. Pregnant personnel should be excluded from handling cytotoxics.
Intravenous administration should be performed using a safe technique to avoid extravasation (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
For instructions on reconstitution of the medicinal product before administration, see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.