DEXILANT DELAYED RELEASE CAPSULE 60mg
1 INDICATIONS AND USAGE
Dexlansoprazole is indicated in adults and in adolescents 12 to 17 years of age for the following:
1.1 Healing of Erosive Esophagitis
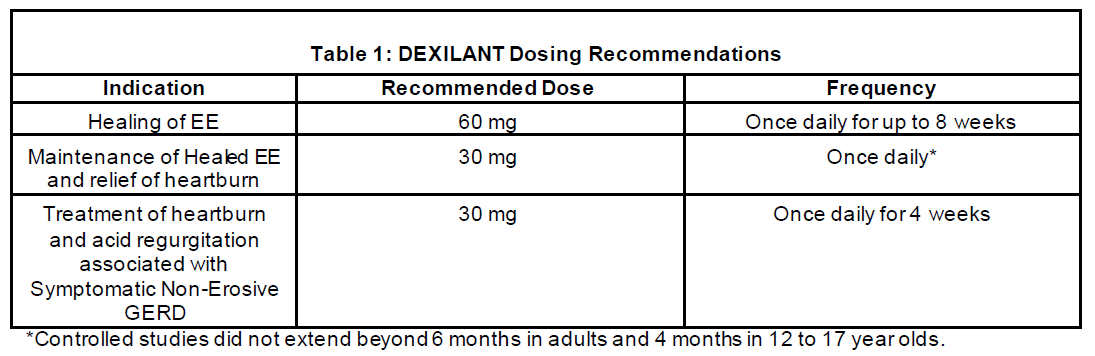
DEXILANT is indicated for healing of all grades of erosive esophagitis (EE) for up to 8 weeks.
1.2 Maintenance of Healed Erosive Esophagitis
DEXILANT is indicated to maintain healing of EE and relief of heartburn for up to 6 months.
1.3 Symptomatic Non-Erosive Gastroesophageal Reflux Disease
DEXILANT is indicated for the treatment of heartburn and acid regurgitation associated with symptomatic non-erosive gastroesophageal reflux disease (GERD) for 4 weeks.
4 CONTRAINDICATIONS
DEXILANT is contraindicated in patients with known hypersensitivity to any component of the formulation [see Description (11) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ]. Hypersensitivity and anaphylaxis have been reported with DEXILANT use [see Adverse Reactions (6.1) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
DEXILANT is available as capsules in 30 mg and 60 mg strengths for adults and in adolescents 12 to 17 years of age. Directions for use in each indication are summarized in Table 1.
2.2 Special Patient Populations
Elderly Patients
No dosage adjustment is necessary for elderly patients.
Pediatric Patients
The safety and efficacy of dexlansoprazole in children under 12 years of age have not been established.
Impaired Renal Function
No dosage adjustment is necessary for patients with renal impairment [see Clinical Pharmacology (11.3) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ].
Hepatic Impairment
No adjustment for DEXILANT is necessary for patients with mild hepatic impairment (Child-Pugh Class A). Consider a maximum daily dose of 30 mg for patients with moderate hepatic impairment (Child-Pugh Class B). No studies have been conducted in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7) and Clinical Pharmacology (11.3) – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information ].
2.3 Important Administration Information
DEXILANT can be taken without regard to food or timing of food.
DEXILANT can be swallowed whole.
DEXILANT should not be chewed.
- For patients who have difficulty swallowing capsules, follow the instructions below for administration:
Administration with Applesauce
- Place one tablespoon into a clean container
- Open capsule
- Sprinkle intact granules on apple sauce
- Swallow applesauce and granules immediately. Do not chew granules. Do not save the applesauce and granules for later use.
Administration with Water in an Oral Syringe
- Open the capsule and empty the granules into a clean container with 20 mL of water.
- Withdraw the entire mixture into a syringe.
- Gently swirl the syringe in order to keep granules from settling.
- Administer the mixture immediately into the mouth. Do not save the water and granule mixture for later use.
- Refill the syringe with 10 mL of water, swirl gently, and administer.
- Refill the syringe again with 10 mL of water, swirl gently, and administer.
Administration with Water via a Nasogastric Tube (≥16 French)
- Open the capsule and empty the granules into a clean container with 20 mL of water.
- Withdraw the entire mixture into a catheter-tip syringe.
- Swirl the syringe gently in order to keep the granules from settling, and immediately inject the mixture through the nasogastric tube into the stomach. Do not save the water and granule mixture for later use.
- Refill the syringe with 10 mL of water, swirl gently, and flush the tube.
- Refill the syringe again with 10 mL of water, swirl gently, and administer.