CRAVIT I.V. For Infusion 5mg/ml
INDICATIONS AND USAGE
CRAVIT® I.V. are indicated for the treatment of adults (≥18 years of age) with mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
CRAVIT® I.V. is indicated when intravenous administration offers a route of administration advantageous to the patient (e.g., patient cannot tolerate an oral dosage form). Please see DOSAGE AND ADMINISTRATION for specific recommendations.
Acute bacterial sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis.
Acute bacterial exacerbation of chronic bronchitis due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Community‐acquired pneumonia due to Staphylococcus aureus, Streptococcus pneumoniae (including penicillin-resistant strains, MIC value for penicillin ≥ 2mcg/mL), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae. (See CLINICAL STUDIES – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.)
Nosocomial pneumonia due to methicillin‐susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, or Streptococcus pneumoniae. Adjunctive therapy should be used as clinically indicated. Where Pseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an anti‐pseudomonal β‐lactam is recommended.
Complicated skin and skin structure infections due to methicillin-sensitive Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes, or Proteus mirabilis.
Uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections due to Staphylococcus aureus, or Streptococcus pyogenes.
Complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa.
Acute pyelonephritis (mild to moderate) caused by Escherichia coli.
Uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus.
Chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis, or methicillin-susceptible Staphylococcus epidermidis.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin. Therapy with levofloxacin may be initiated before results of these tests are known; once results become available, appropriate therapy should be selected.
As with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with levofloxacin. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.
CONTRAINDICATIONS
Levofloxacin is contraindicated in persons with a history of hypersensitivity to levofloxacin, quinolone antimicrobial agents, or any other components of this product.
DOSAGE AND ADMINISTRATION
CRAVIT® I.V. should only be administered by intravenous infusion. It is not for intramuscular, intrathecal, intraperitioneal, or subcutaneous administration.
CAUTION: RAPID OR BOLUS INTRAVENOUS INFUSION MUST BE AVOIDED. Levofloxacin Injection should be infused intravenously slowly over a period of not less than 60 or 90 minutes, depending on the dosage. (See PRECAUTIONS – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.)
The usual dose of CRAVIT® I.V. is 250 mg or 500 mg administered orally or by slow infusion over 60 minutes every 24 hours or 750 mg administered by slow infusion over 90 minutes every 24 hours, as indicated by infection and described in the following dosing chart. These recommendations apply to patients with normal renal function (i.e., creatinine clearance >80 mL/min). For patients with altered renal function see the Patients with Impaired Renal Function subsection.
Patients with Normal Renal Function
Infection*Unit DoseFreq.Duration**Daily DoseAcute Bacterial Exacerbation of Chronic Bronchitis500 mgq24h7 days500 mgCommunity‐Acquired Pneumonia500 mgq24h7–14 days500 mgNosocomial Pneumonia750 mgq24h7–14 days750 mgAcute Bacterial Sinusitis500 mgq24h10–14 days500 mgComplicated SSSI750 mgq24h7–14 days750 mgUncomplicated SSSI500 mgq24h7–10 days500 mgComplicated UTI250 mgq24h10 days250 mgAcute pyelonephritis250 mgq24h10 days250 mgUncomplicated UTI250 mgq24h3 days250 mgChronic Bacterial Prostatitis500 mgq24h28 days500 mg
*DUE TO THE DESIGNATED PATHOGENS (See INDICATIONS AND USAGE.)
\\ Sequential therapy (intravenous to oral) may be instituted at the discretion of the physician.
Patients with Impaired Renal Function
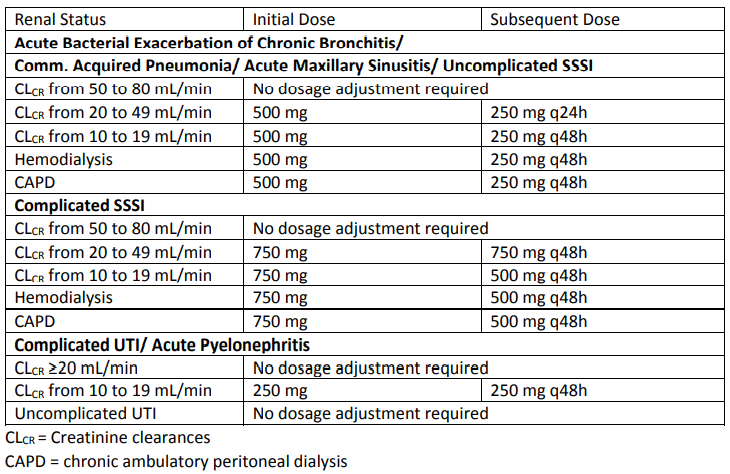
When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance.
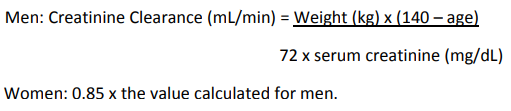
The serum creatinine should represent a steady state of renal function.
Stability of CRAVIT® I.V. as Supplied
When stored under recommended conditions, CRAVIT® I.V., as supplied in 50 mL and 100 mL vials, is stable through the expiration date printed on the label.