ENHERTU POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION 100MG/VIAL
4.1 Therapeutic indications
Metastatic Breast Cancer
HER2-Positive
ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen.
HER2-Low
ENHERTU as monotherapy is indicated for the treatment of adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received at least one prior line of chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy.
Patients with hormone receptor positive (HR+) breast cancer should have received at least one and be no longer considered eligible for endocrine therapy.
Unresectable or Metastatic Non-Small Cell Lung Cancer (NSCLC)
ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2 (ERBB2) mutations and who have received a prior systemic therapy.
Locally Advanced or Metastatic Gastric Cancer
ENHERTU is indicated for the treatment of adult patients with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received two or more prior regimens, including a trastuzumab-based regimen.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
4.2 Posology and method of administration
In order to prevent medicinal product errors, it is important to check the vial labels to ensure that the medicinal product being prepared and administered is ENHERTU (trastuzumab deruxtecan) and not trastuzumab or trastuzumab emtansine.
Do not substitute ENHERTU for or with trastuzumab or trastuzumab emtansine.
Patient Selection for HER2-low Metastatic Breast Cancer
Select patients for treatment of unresectable or metastatic HER2-low breast cancer based on IHC 1+ or IHC 2+/ISH- tumor status.
Patient Selection for Unresectable or Metastatic NSCLC
Select patients for the treatment of unresectable or metastatic NSCLC with ENHERTU based on the presence of activating HER2 (ERBB2) mutations detected by a validated test.
Premedication
ENHERTU is emetogenic (see section 4.7 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information), which includes delayed nausea and/or vomiting. Prior to each dose of ENHERTU, patients should be premedicated with a combination regimen of two or three medicinal products (e.g., dexamethasone with either a 5-HT3 receptor antagonist and/or an NK1 receptor antagonist, as well as other medicinal products as indicated) for prevention of chemotherapy-induced nausea and vomiting.
Posology
The initial dose should be administered as a 90-minute intravenous infusion. If the prior infusion was well tolerated, subsequent doses of ENHERTU may be administered as 30-minute infusions.
The infusion rate of ENHERTU should be slowed or interrupted if the patient develops infusion-related symptoms. ENHERTU should be permanently discontinued in case of severe infusion reactions.
Metastatic Breast Cancer
The recommended dose of ENHERTU is 5.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity.
Unresectable or Metastatic NSCLC
The recommended dose of ENHERTU is 5.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity.
Locally Advanced or Metastatic Gastric Cancer
The recommended dose of ENHERTU is 6.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity.
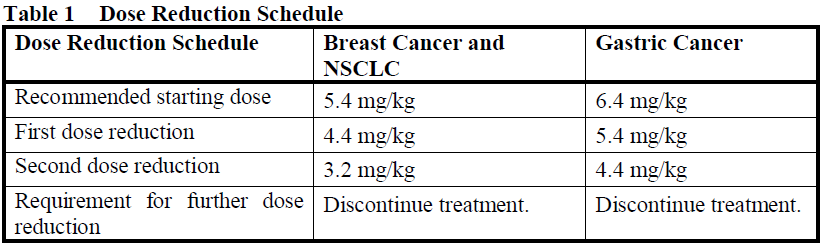
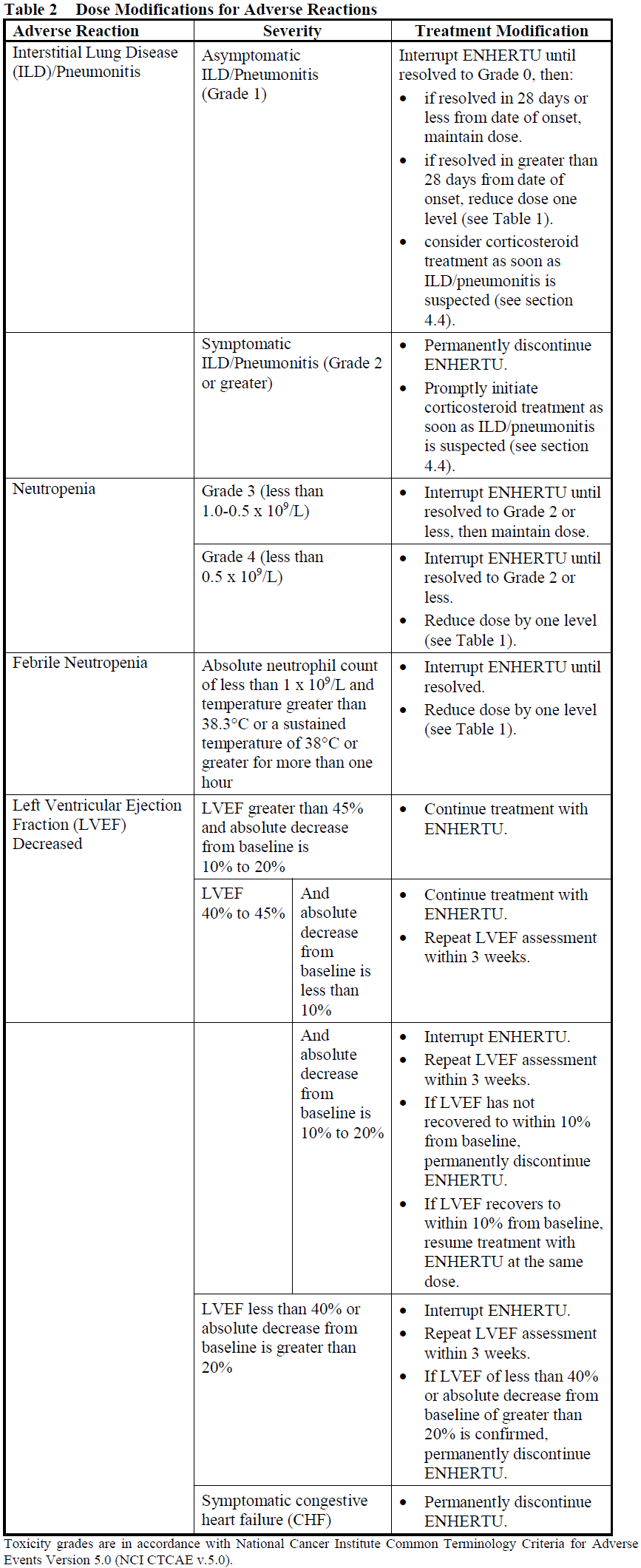
Dose Modifications
Management of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of ENHERTU per guidelines provided in Tables 1 and 2.
ENHERTU dose should not be re-escalated after a dose reduction is made.
Delayed or Missed Dose
If a planned dose is delayed or missed, it should be administered as soon as possible without waiting until the next planned cycle. The schedule of administration should be adjusted to maintain a 3-week interval between doses. The infusion should be administered at the dose and rate the patient tolerated in the most recent infusion.
Special Populations
Geriatrics
No dose adjustment of ENHERTU is required in patients aged 65 years or older.
Of the 1449 patients in clinical studies across multiple tumor types treated with ENHERTU 5.4 mg/kg, 24.2% were 65 years or older and 4.3% were 75 years or older. The incidence of Grade 3–4 adverse reactions observed was 49.7% in patients aged 65 years or older and 42.0% in younger patients. There was a higher incidence of Grade 3–4 adverse reactions observed in patients aged 65 years or older (55.1%) as compared to younger patients (48.9%) in patients with HER2-positive breast cancer.
Of the 669 patients in clinical studies across multiple tumor types treated with ENHERTU 6.4 mg/kg, 39.2% were 65 years or older and 7.6% were 75 years or older. The incidence of Grade 3–4 adverse reactions observed was 59.9% in patients aged 65 years or older and 62.2% in younger patients. There was a higher incidence of ≥Grade 3 adverse reactions observed in younger patients (87%) as compared to patients aged 65 years or older (76%) in patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma in DESTINY-Gastric01.
Population pharmacokinetic analysis indicates that age does not have a clinically meaningful effect on the pharmacokinetics of trastuzumab deruxtecan.
Pediatrics
The safety and efficacy in children and adolescents below 18 years of age have not been established as there is no relevant use in the pediatric population.
Renal Impairment
No dose adjustment is required in patients with mild (creatinine clearance [CLcr] ≥60 and <90 mL/min) or moderate (CLcr ≥30 and <60 mL/min) renal impairment. Limited data are available in patients with severe renal impairment. A higher incidence of Grade 1 and 2 ILD/pneumonitis leading to an increase in discontinuation of therapy has been observed in patients with moderate renal impairment. Patients with moderate or severe renal impairment should be monitored carefully (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic Impairment
No dose adjustment is required in patients with mild (total bilirubin ≤ULN and any AST >ULN or total bilirubin >1 to 1.5 times ULN and any AST) hepatic impairment. There are insufficient data to make a recommendation on dose adjustment in patients with moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment. No data are available in patients with severe (total bilirubin >3 to 10 times ULN and any AST) hepatic impairment.
Method of Administration
ENHERTU is for intravenous use. It must be reconstituted and diluted by a healthcare professional and administered as an intravenous infusion. ENHERTU must not be administered as an intravenous push or bolus.
For instructions on reconstitution and dilution of ENHERTU before administration, see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.