INFANRIX HEXA VACCINE
Indications
Infanrix hexa is indicated for primary and booster vaccination of infants and toddlers against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis and Haemophilus influenzae type b.
The use of Infanrix hexa should be in accordance with official recommendations.
Contraindications
Hypersensitivity to the active substances or to any of the excipients or residues such as formaldehyde, neomycin and polymyxin (see Qualitative and quantitative composition and List of Excipients – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hypersensitivity after previous administration of diphtheria, tetanus, pertussis, hepatitis B, polio or Hib vaccines.
Infanrix hexa is contraindicated if the child has experienced an encephalopathy of unknown aetiology, occurring within 7 days following previous vaccination with pertussis-containing vaccine. In these circumstances, pertussis vaccination should be discontinued and the vaccination course should be continued with diphtheria-tetanus, hepatitis B, inactivated polio and Hib vaccines.
Dosage and Administration
Posology
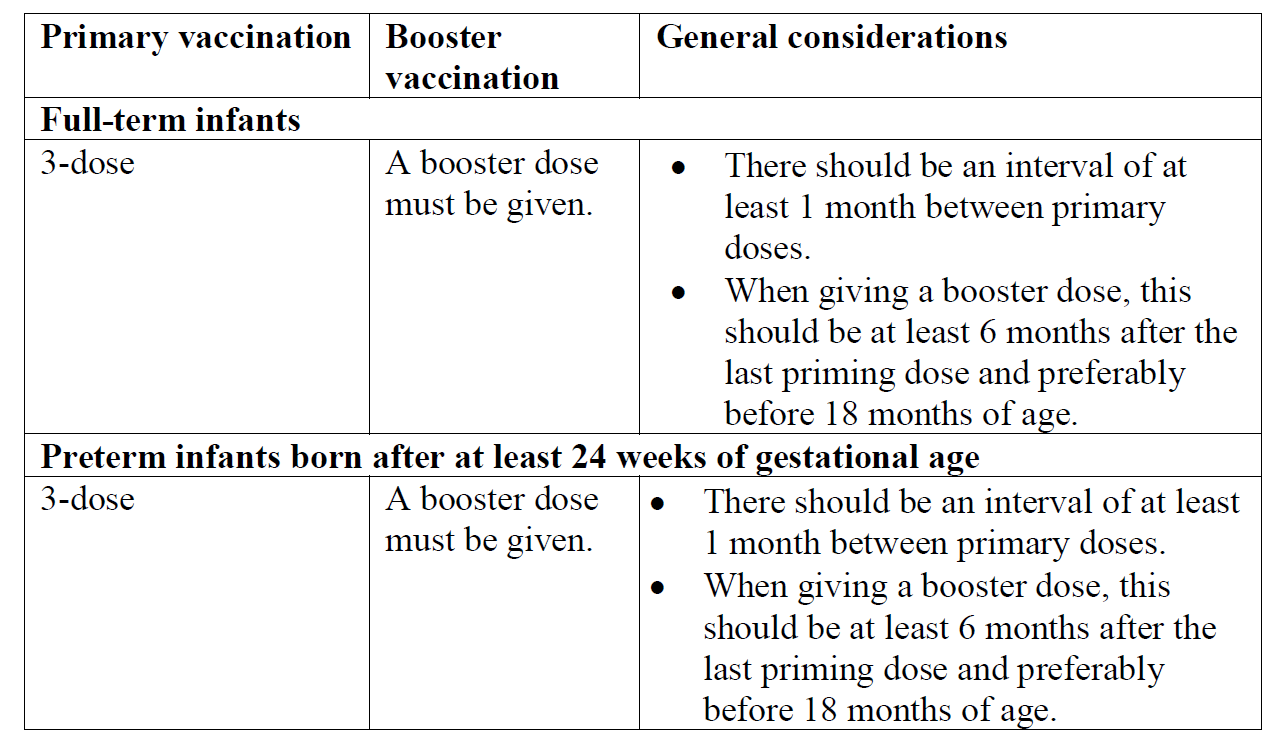
The primary vaccination schedule consists of three doses (of 0.5 ml) which should be administered according to official recommendations (see Pharmacodynamics for schedules evaluated in clinical trials – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). Infanrix hexa can be considered for the booster if the antigen composition is in accordance with the official recommendations.
The vaccine may be used to complete a vaccination course initiated with Infanrix-IPV+Hib vaccine.
During the development of the vaccine, the following primary vaccination schedules which are compatible with a policy of administering hepatitis B vaccine at birth were studied in full-term infants: at 2, 3, 4 months of age; at 2, 4, 6 months of age; at 1.5, 3, 5 months of age and at 6, 10, 14 weeks of age.
Additionally, in a study in full-term infants undertaken in Singapore, Infanrix hexa was given as part of a 3, 4, 5 month schedule with Infanrix-IPV+Hib. The Infanrix-IPV+Hib was administered at months 3 and 4 and Infanrix hexa given at month 5. As part of this study, monovalent Hepatitis B vaccine was given at 0 and 1 month in accord with Singapore’s Hepatitis B immunisation policy.
Immunogenicity data for preterm infants was solely obtained in trials that studied 2-4-6 months schedule for primary vaccination. Safety data for preterm infants was also obtained in study Rota-54 that included a greater variety of schedules, i.e. in France (2-3-4 months), Poland (2-3/4-5 months), Spain and Portugal (2-4-6 months).
The Expanded Program on Immunisation schedule (at 6, 10, 14 weeks of age) may only be used if a dose of hepatitis B vaccine has been given at birth.
Where a dose of hepatitis B vaccine is given at birth, Infanrix hexa can be used as a replacement for supplementary doses of hepatitis B vaccine from the age of 6 weeks. If a second dose of hepatitis B vaccine is required before this age, monovalent hepatitis B vaccine should be used.
Locally established immunoprophylactic measures against hepatitis B should be maintained.
Method of administration
Infanrix hexa is for deep intramuscular injection.