WEGOVY 2.4 MG/DOSE FLEXTOUCH SOLUTION FOR INJECTION IN PRE-FILLED PEN 3.2 MG/ML
4.1 Therapeutic indications
Wegovy® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of
- ≥30 kg/m2 (obesity), or
- ≥27 kg/m2 to <30 kg/m2 (overweight) in the presence of at least one weight-related comorbidity e.g. dysglycaemia (prediabetes or type 2 diabetes mellitus), hypertension, dyslipidaemia, obstructive sleep apnoea or cardiovascular disease.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
4.2 Posology and method of administration
Posology
The maintenance dose of semaglutide 2.4 mg once-weekly is reached by starting with a dose of 0.25 mg. To reduce the likelihood of gastrointestinal symptoms, the dose should be escalated over a 16-week period to a maintenance dose of 2.4 mg once weekly (see Table 1). In case of significant gastrointestinal symptoms, consider delaying dose escalation or lowering to the previous dose until symptoms have improved.
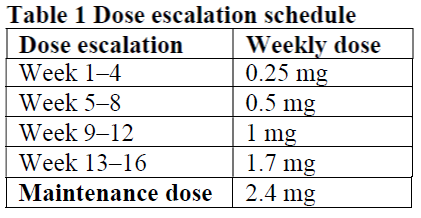
Weekly doses higher than 2.4 mg are not recommended.
Patients with type 2 diabetes
When initiating semaglutide in patients with type 2 diabetes, consider reducing the dose of concomitantly administered insulin or insulin secretagogues (such as sulfonylureas) to reduce the risk of hypoglycaemia, see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
Missed dose
If a dose is missed, it should be administered as soon as possible and within 5 days after the missed dose. If more than 5 days have passed, the missed dose should be skipped, and the next dose should be administered on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule. If more doses are missed, reducing the starting dose for re-initiation should be considered.
Special populations
Elderly (≥65 years old)
No dose adjustment is required based on age. Therapeutic experience in patients ≥75 years of age is limited, and greater sensitivity of some older individuals cannot be excluded.
Patients with renal impairment
No dose adjustment is required for patients with mild or moderate renal impairment. Experience with the use of semaglutide in patients with severe renal impairment is limited. Semaglutide is not recommended for use in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) including patients with end-stage renal disease (see sections 4.4, 4.8 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Patients with hepatic impairment
No dose adjustment is required for patients with mild or moderate hepatic impairment. Experience with the use of semaglutide in patients with severe hepatic impairment is limited. Semaglutide is not recommended for use in patients with severe hepatic impairment and should be used cautiously in patients with mild or moderate hepatic impairment (see sections 4.4 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric population
The safety and efficacy of semaglutide in children and adolescents below 18 years have not yet been established. No data are available.
Method of administration
Subcutaneous use.
Wegovy® is administered once weekly at any time of the day, with or without meals.
It is to be injected subcutaneously in the abdomen, in the thigh or in the upper arm. The injection site can be changed. It should not be administered intravenously or intramuscularly.
The day of weekly administration can be changed if necessary, as long as the time between two doses is at least 3 days (>72 hours). After selecting a new dosing day, once-weekly dosing should be continued.
Patients should be advised to read the instruction for use included in the package leaflet carefully before administering the medicinal product.
For further information before administration see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.