NIMENRIX POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
4.1. Indications
Nimenrix™ is indicated for active immunization of individuals from 6 weeks of age against invasive meningococcal diseases caused by Neisseria meningitidis groups A, C, W-135 and Y.
4.3. Contraindications
Nimenrix™ should not be administered to subjects with hypersensitivity to the active substances or to any of the excipients contained in the vaccine (see sections 2 and 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
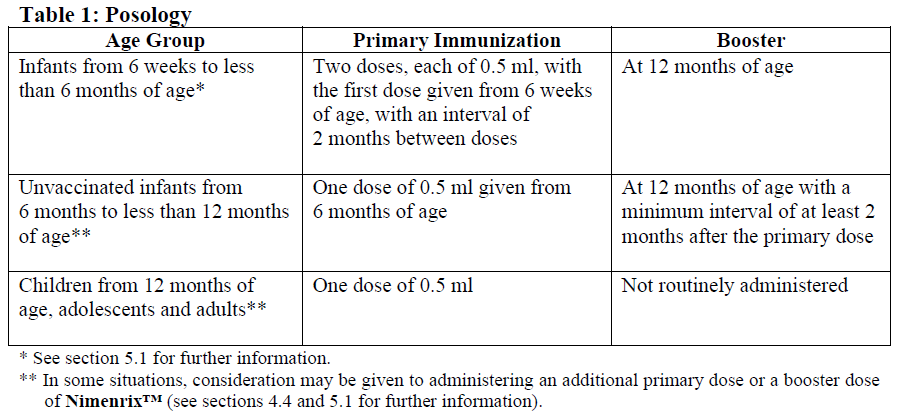
4.2. Dosage and Administration
Posology
Nimenrix™ should be used in accordance with available official recommendations.
Previously vaccinated children from 12 months of age, adolescents and adults
Nimenrix™ may be given as a booster dose to individuals who have previously received primary vaccination with a conjugated or plain polysaccharide meningococcal vaccine (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Special populations
Individuals who have underlying conditions predisposing them to meningococcal infection due to anatomic or functional asplenia (such as sickle cell disease) may receive at least one dose of Nimenrix™ (see sections 4.8 and 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Method of administration
Immunization should be carried out by intramuscular injection only.
In infants, the recommended injection site is the anterolateral aspect of the thigh.
In individuals from 1 year of age, the recommended injection site is the anterolateral aspect of the thigh or the deltoid muscle (see sections 4.4 and 4.5 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
For instructions on reconstitution of the medicinal product before administration, see section 6.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.