LEVEMIR® FLEXPEN® 100U/ML, 3ML
Therapeutic indications
Treatment of diabetes mellitus in adults, adolescents and children aged 2 years and above.
Contraindications
Hypersensitivity to the active substance or to any of the excipients (see List of excipients – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Posology
Levemir® is a soluble, basal insulin analogue with a prolonged duration of effect (up to 24 hours).
Levemir® can be used alone as the basal insulin or in combination with bolus insulin. It can also be used in combination with oral antidiabetic medicinal products and/or GLP-1 receptor agonists.
Dosage
When Levemir® is used in combination with oral antidiabetic medicinal products or when added to GLP-1 receptor agonists, it is recommended to use Levemir® once daily, initially at a dose of 0.1–0.2 units/kg, or of 10 units in adult patients. The dose of Levemir® should be titrated based on the individual patient's needs.
When a GLP-1 receptor agonist is added to Levemir®, it is recommended to reduce the dose of Levemir® by 20% to minimise the risk of hypoglycaemia. Subsequently, dosage should be adjusted individually.
For individual dose adjustments, the following two titration guidelines are recommended for adults:
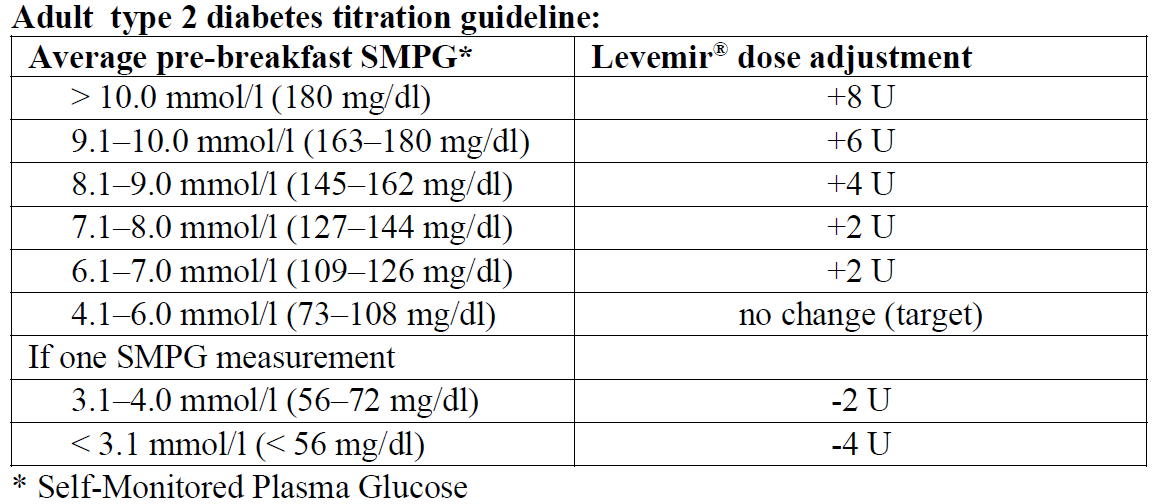
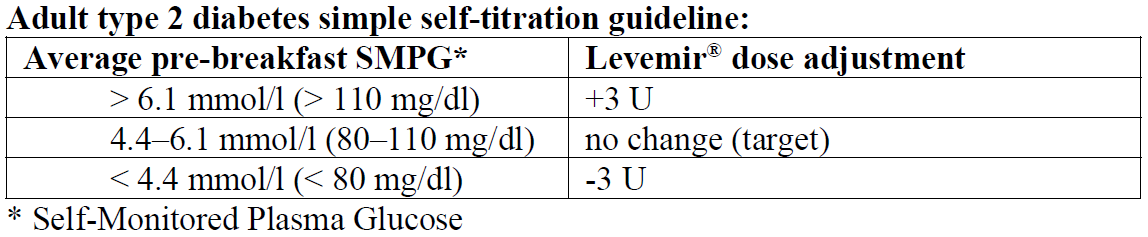
When Levemir® is used as part of a basal-bolus insulin regimen, Levemir® should be administered once or twice daily depending on the patient's needs. The dose of Levemir® should be adjusted individually. For patients who require twice-daily dosing to optimise blood glucose control, the evening dose can be administered in the evening or at bedtime. Adjustment of dose may be necessary if patients undertake increased physical activity, change their usual diet or during concomitant illness. When adjusting dose in order to improve glucose control, patients should be advised to be aware of signs of hypoglycaemia.
Special populations
As with all insulin products, in elderly patients and patients with renal or hepatic impairment, glucose monitoring should be intensified and the Levemir® dosage adjusted on an individual basis.
Paediatric population
The efficacy and safety of Levemir® were demonstrated in adolescents and children aged 2 years and above in studies up to 12 months (see Pharmacodynamic properties – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
In children and adolescents, glucose monitoring should be intensified and the Levemir® dose adjusted on an individual basis.
Levemir® has not been studied in children below the age of 2 years.
Transfer from other insulin products
Transfer to Levemir® from intermediate or long-acting insulin products may require adjustment of dose and timing of administration (see Special warnings and precautions for use – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
As with all insulin products, close glucose monitoring is recommended during the transfer and in the initial weeks thereafter.
Concomitant antidiabetic treatment may need to be adjusted (dose and/or timing of oral antidiabetic medicines or concurrent short-acting insulin products).
Method of administration
Levemir® is for subcutaneous administration only. Levemir® must not be administered intravenously, as it may result in severe hypoglycaemia. Intramuscular administration should also be avoided. Levemir® is not to be used in insulin infusion pumps.
Levemir® is administered subcutaneously by injection in the abdominal wall, the thigh, the upper arm, the deltoid region or the gluteal region. Injection sites should always be rotated within the same region in order to reduce the risk of lipodystrophyy and cutaneous amyloidosis (see Special warnings and precautions for use and Undesirable effects – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information). As with all insulin products, the duration of action will vary according to the dose, injection site, blood flow, temperature and level of physical activity. Levemir® FlexPen® is a pre-filled pen designed to be used with NovoFine® or NovoTwist® disposable needles up to a length of 8 mm. FlexPen® delivers 1–60 units in increments of 1 unit.
Levemir® FlexPen® is colour-coded and accompanied by a package leaflet with detailed instructions for use to be followed.