MAYZENT FILM-COATED TABLET 0.25MG
4.1 Therapeutic indications
Mayzent is indicated for the treatment of adult patients with secondary progressive multiple sclerosis (SPMS) with active disease evidenced by relapses or imaging features of inflammatory activity (see section 5.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.3 Contraindications
- Hypersensitivity to the active substance, or to peanut, soya or any of the excipients listed in section 6.1 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information.
- Immunodeficiency syndrome.
- History of progressive multifocal leukoencephalopathy or cryptococcal meningitis.
- Active malignancies.
- Severe liver impairment (Child-Pugh class C).
- Patients who in the previous 6 months had a myocardial infarction (MI), unstable angina pectoris, stroke/transient ischaemic attack (TIA), decompensated heart failure (requiring inpatient treatment), or New York Heart Association (NYHA) class III/IV heart failure (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
- Patients with a history of second-degree Mobitz type II atrioventricular (AV) block, third-degree AV block, sino-atrial heart block or sick-sinus syndrome, if they do not wear a pacemaker (see section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
- Patients homozygous for CYP2C9*3 (CYP2C9*3*3) genotype (poor metaboliser).
- During pregnancy and in women of childbearing potential not using effective contraception (see sections 4.4 and 4.6 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
4.2 Posology and method of administration
Before initiation of treatment, patients must be genotyped for CYP2C9 to determine their CYP2C9 metaboliser status (see sections 4.4, 4.5 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
In patients with a CYP2C9*3*3 genotype, Mayzent should not be used (see sections 4.3, 4.4 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
In patients with a CYP2C9*2*3 or *1*3 genotype, the recommended maintenance dose is 1 mg taken once daily (four tablets of 0.25 mg) (see sections 4.4 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
The recommended maintenance dose of Mayzent in all other CYP2C9 genotype patients is 2 mg. Mayzent is taken once daily.
Posology
Treatment initiation
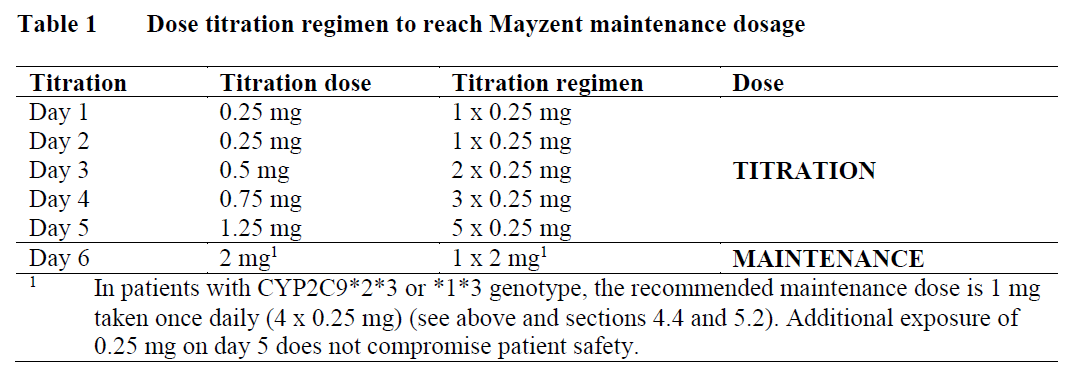
Treatment has to be started with a titration pack that lasts for 5 days. Treatment starts with 0.25 mg once daily on days 1 and 2, followed by once-daily doses of 0.5 mg on day 3, 0.75 mg on day 4, and 1.25 mg on day 5, to reach the patient’s prescribed maintenance dose of Mayzent starting on day 6 (see Table 1). During the first 6 days of treatment initiation the recommended daily dose should be taken once daily in the morning with or without food.
Missed dose(s) during treatment initiation
During the first 6 days of treatment, if a titration dose is missed on one day, treatment needs to be re-initiated with a new titration pack.
Missed dose after day 6
If a dose is missed, the prescribed dose should be taken at the next scheduled time; the next dose should not be doubled.
Re-initiation of maintenance therapy after treatment interruption
If maintenance treatment is interrupted for 4 or more consecutive daily doses, Mayzent needs to be re-initiated with a new titration pack.
Special populations
Elderly
Mayzent has not been studied in patients aged 65 years and above. Clinical studies included patients up to the age of 61 years. Mayzent should be used with caution in the elderly due to insufficient data on safety and efficacy (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Renal impairment
Based on clinical pharmacology studies, no dose adjustment is needed in patients with renal impairment (see section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic impairment
Mayzent must not be used in patients with severe hepatic impairment (Child-Pugh class C) (see section 4.3). Although no dose adjustment is needed in patients with mild or moderate hepatic impairment, caution should be exercised when initiating treatment in these patients (see sections 4.4 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric population
The safety and efficacy of Mayzent in children and adolescents aged 0 to 18 years have not yet been established. No data are available.
Method of administration
Oral use. Mayzent is taken with or without food.
The film-coated tablets should be swallowed whole with water.