KOSELUGO HARD CAPSULES 25MG
4.1 Therapeutic indications
KOSELUGO is indicated for the treatment of paediatric patients aged 3 years and above with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN).
4.3 Contraindications
None.
4.2 Posology and method of administration
Therapy should be initiated by a physician experienced in the diagnosis and the treatment of patients with NF1-related tumours.
Posology
The recommended dose of KOSELUGO is 25 mg/m2 of body surface area (BSA), taken orally twice daily (approximately every 12 hours).
Dosing is individualised based on BSA (mg/m2) and rounded to the nearest achievable 5 mg or 10 mg dose (up to a maximum single dose of 50 mg). Different strengths of KOSELUGO capsules can be combined to attain the desired dose (Table 1).
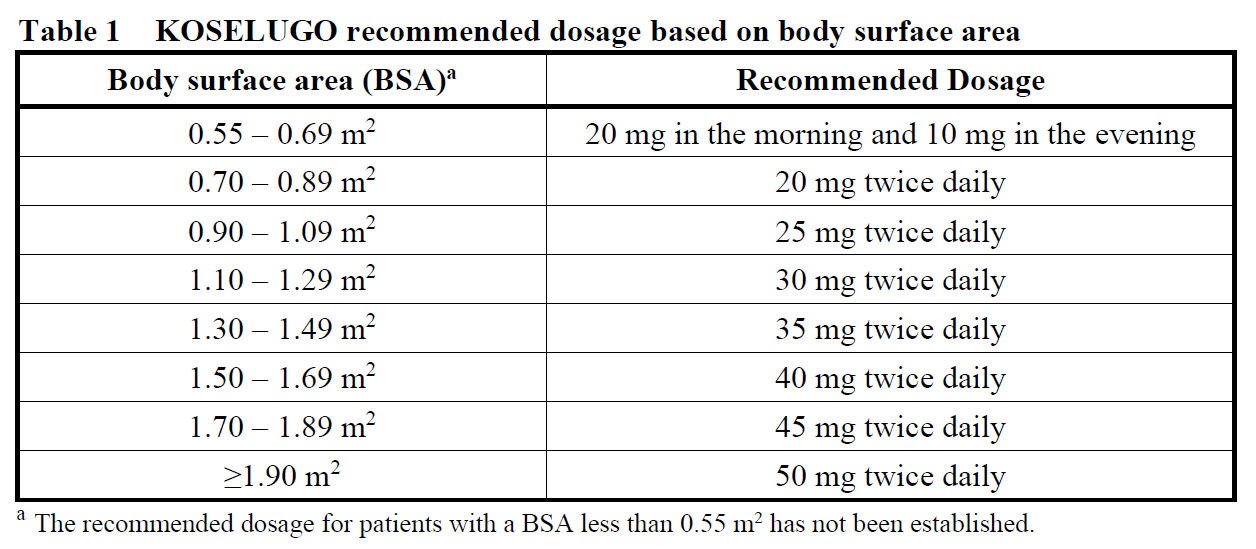
Treatment with KOSELUGO should continue as long as clinical benefit is observed, or until PN progression or the development of unacceptable toxicity.
Method of administration
KOSELUGO should be taken on an empty stomach with no food or drink other than water. Do not consume food 2 hours prior to dosing and 1 hour after dosing (see Sections 4.5 and 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
KOSELUGO capsules should be swallowed whole with water, and should not be chewed, dissolved, or opened.
Missed dose
If a dose of KOSELUGO is missed, it should only be taken if it is more than 6 hours until the next scheduled dose.
Vomiting
Do not take an additional dose if vomiting occurs after KOSELUGO administration but continue with the next scheduled dose.
Dose adjustments
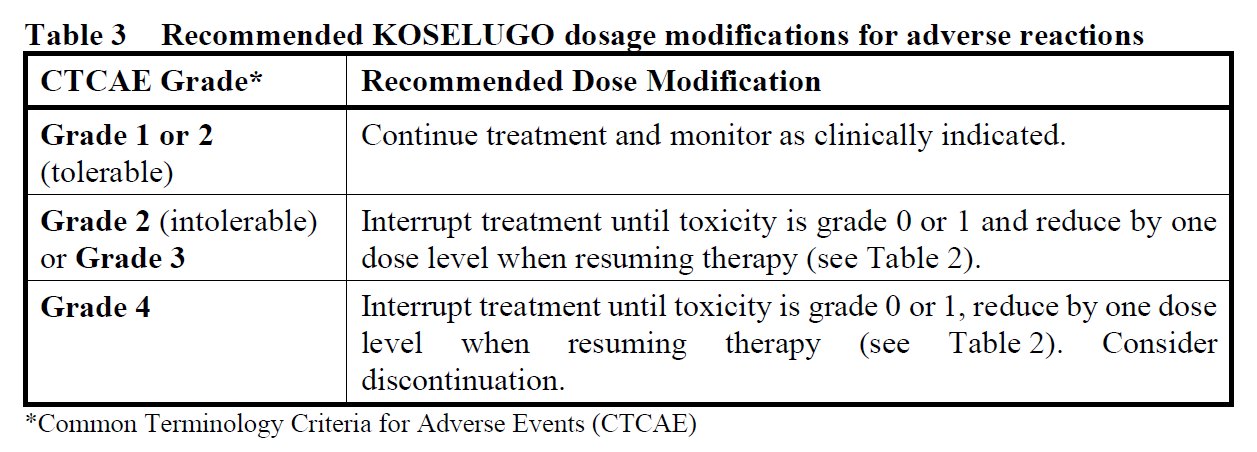
For adverse events
Interruption and/or dose reduction or permanent discontinuation of KOSELUGO may be required based on individual safety and tolerability (see Sections 4.4 and 4.8 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Recommended dose reductions are given in Table 2 and may require the daily dose to be divided into two administrations of different strength or for treatment to be given as a once daily dose.
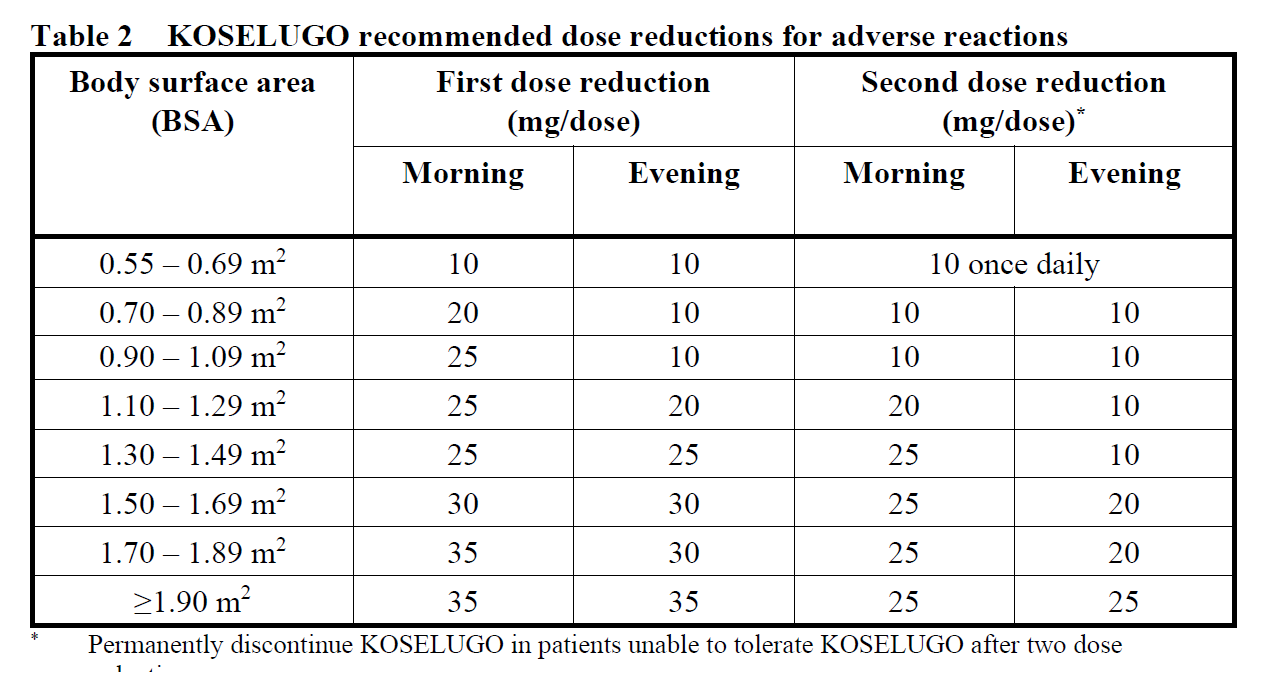
Dose modification advice for left ventricular ejection fraction (LVEF) reduction
In cases of asymptomatic LVEF reduction of ≥10 percentage points from baseline and below the institutional lower level of normal (LLN), KOSELUGO treatment should be interrupted until resolution. Once resolved, reduce KOSELUGO by one dose level when resuming therapy (see Table 2).
In patients who develop symptomatic LVEF reduction or a grade 3 or 4 LVEF reduction, KOSELUGO should be discontinued and a prompt cardiology referral should be carried out (see Section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Dose modification advice for ocular toxicities
KOSELUGO treatment should be interrupted in patients diagnosed with retinal pigment epithelial detachment (RPED) or central serous retinopathy (CSR) with reduced visual acuity until resolution; reduce KOSELUGO by one dose level when resuming therapy (see Table 2). In patients diagnosed with RPED or CSR without reduced visual acuity, ophthalmic assessment should be conducted every 3 weeks until resolution. In patients who are diagnosed with retinal vein occlusion (RVO), treatment with KOSELUGO should be permanently discontinued (see Section 4.4 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Dose adjustments for co-administration with CYP3A4 or CYP2C19 inhibitors
Concomitant use of strong or moderate CYP3A4 or CYP2C19 inhibitors is not recommended and alternative agents should be considered. If a strong or moderate CYP3A4 or CYP2C19 inhibitor must be co-administered, the recommended KOSELUGO dose reduction is as follows: If a patient is currently taking 25 mg/m2 twice daily, dose reduce to 20 mg/m2 twice daily. If a patient is currently taking 20 mg/m2 twice daily, dose reduce to 15 mg/m2 twice daily (see Table 4 and section 4.5).
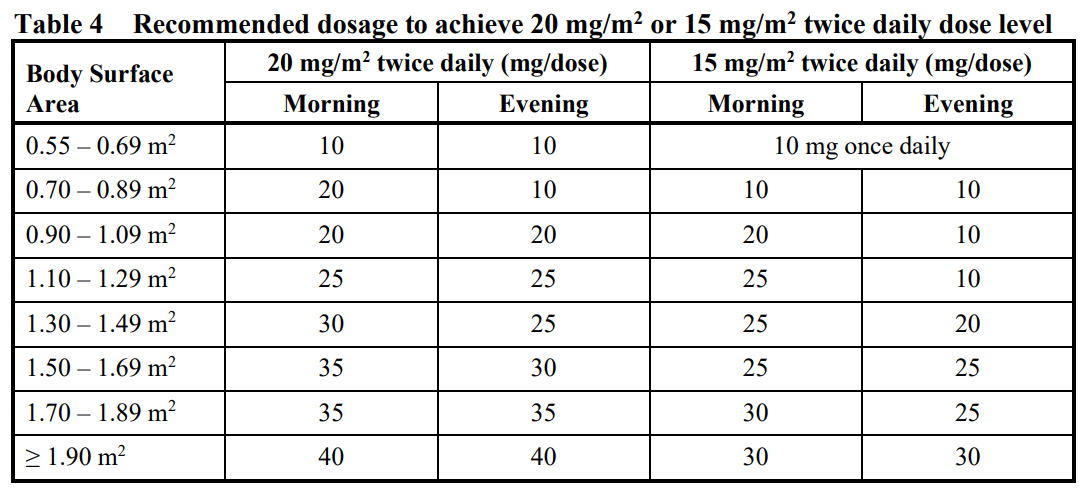
Special patient populations
Renal impairment
Based on clinical studies no dose adjustment is recommended in patients with mild, moderate, severe renal impairment or those with End Stage Renal Disease (ESRD) (see Section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Hepatic impairment
Based on clinical studies, no dose adjustment is recommended in patients with mild hepatic impairment. The starting dose should be reduced in patients with moderate hepatic impairment to 20 mg/m2 BSA, twice daily (see Table 4). KOSELUGO is not recommended for use in patients with severe hepatic impairment (see Section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Ethnicity
Increased systemic exposure has been seen in adult Asian subjects, although there is considerable overlap with Western subjects when corrected for body weight. No specific adjustment to the starting dose is recommended for paediatric Asian patients, however, these patients should be closely monitored for adverse events (see Section 5.2 – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information).
Paediatric population
The safety and efficacy of KOSELUGO in children less than 3 years of age has not been established. No data are currently available.